1. A cycle consisting of one constant pressure, one constant volume and two isentropic processes is known as the: a) Carnot cycle 2. The
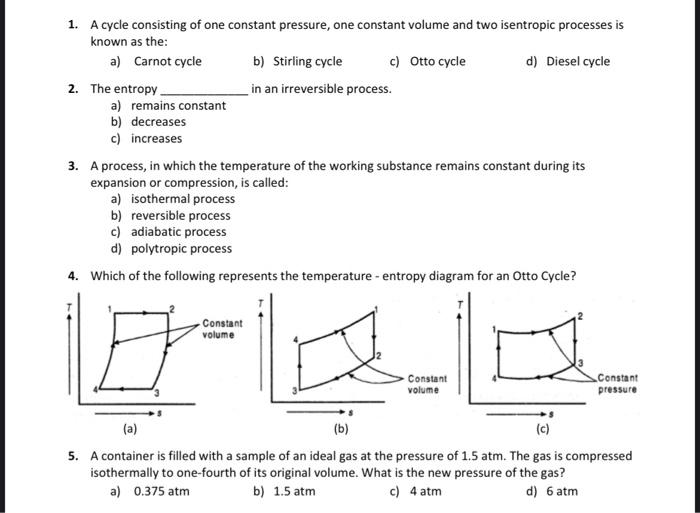
1. A cycle consisting of one constant pressure, one constant volume and two isentropic processes is known as the: a) Carnot cycle 2. The entropy. a) remains constant b) decreases c) increases b) Stirling cycle in an irreversible process. c) Otto cycle Constant volume 3. A process, in which the temperature of the working substance remains constant during its expansion or compression, is called: a) isothermal process b) reversible process c) adiabatic process d) polytropic process 4. Which of the following represents the temperature - entropy diagram for an Otto Cycle? d) Diesel cycle Constant volume Constant pressure (a) (b) (c) 5. A container is filled with a sample of an ideal gas at the pressure of 1.5 atm. The gas is compressed isothermally to one-fourth of its original volume. What is the new pressure of the gas? a) 0.375 atm b) 1.5 atm c) 4 atm d) 6 atm
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


