Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. (a) Define what is meant by heat and work in thermodynamics. (b) Define what is meant by an adiabatic process. Show that in
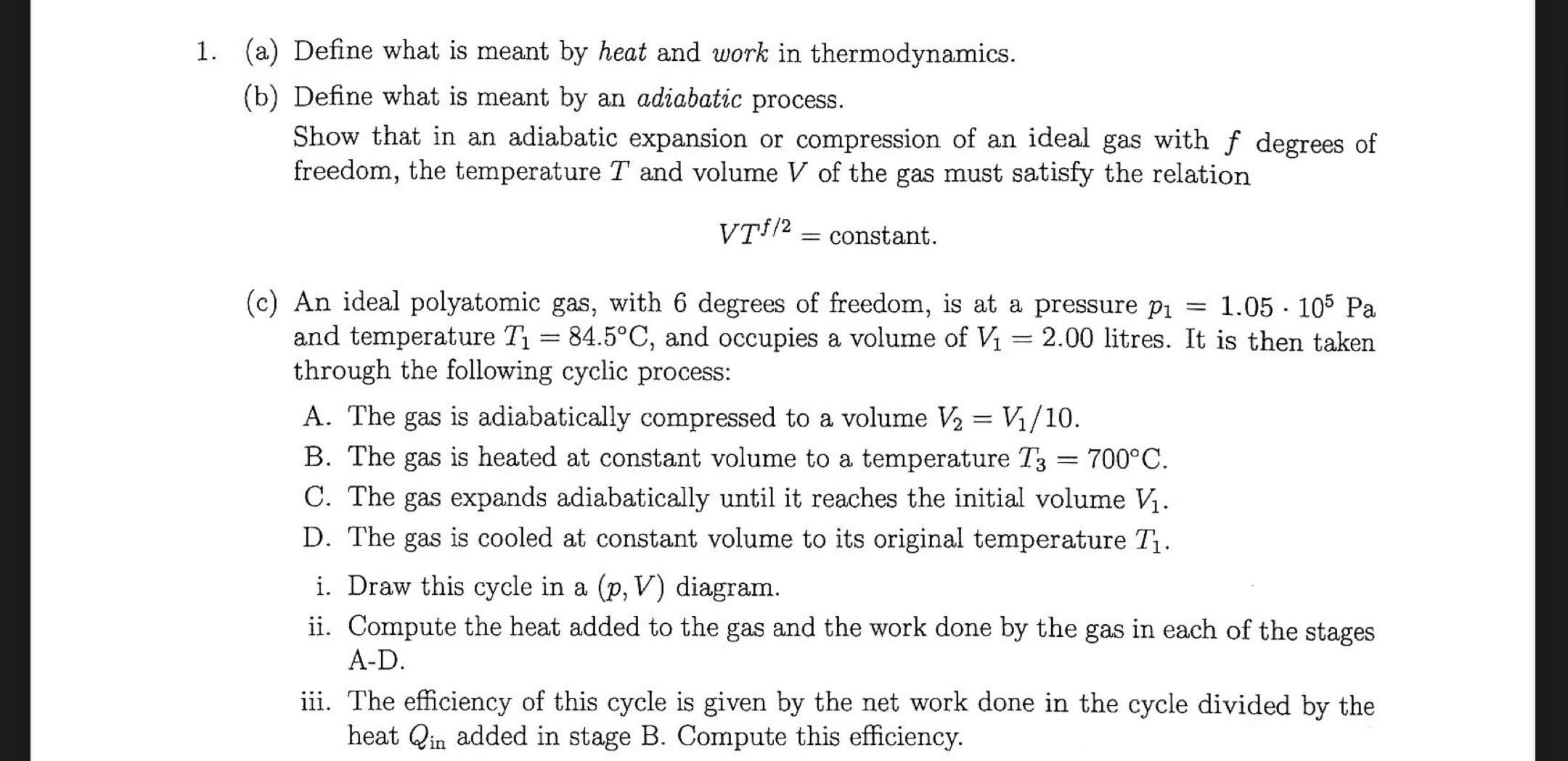
1. (a) Define what is meant by heat and work in thermodynamics. (b) Define what is meant by an adiabatic process. Show that in an adiabatic expansion or compression of an ideal gas with f degrees of freedom, the temperature T and volume V of the gas must satisfy the relation VTf/2 = constant. - (c) An ideal polyatomic gas, with 6 degrees of freedom, is at a pressure p = 1.05 105 Pa and temperature T = 84.5C, and occupies a volume of V 84.5C, and occupies a volume of V = 2.00 litres. It is then taken through the following cyclic process: - - A. The gas is adiabatically compressed to a volume V = V/10. B. The gas is heated at constant volume to a temperature T3 = : 700C. C. The gas expands adiabatically until it reaches the initial volume V. D. The gas is cooled at constant volume to its original temperature T. i. Draw this cycle in a (p, V) diagram. ii. Compute the heat added to the gas and the work done by the gas in each of the stages A-D. iii. The efficiency of this cycle is given by the net work done in the cycle divided by the heat Qin added in stage B. Compute this efficiency.
Step by Step Solution
★★★★★
3.40 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 The first law of thermodynamics asserts the conservation of the systems total energ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


