Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A metal cube has an edge length of 16.0 mm and a mass of 36.7 g. Calculate the density of the metal. Show
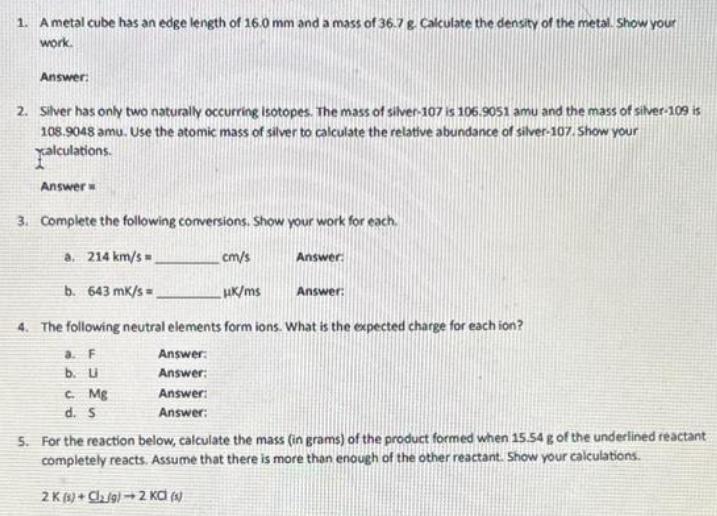
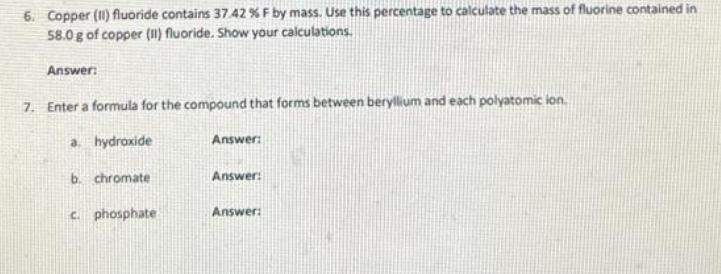
1. A metal cube has an edge length of 16.0 mm and a mass of 36.7 g. Calculate the density of the metal. Show your work. Answer: 2. Silver has only two naturally occurring isotopes. The mass of silver-107 is 106.9051 amu and the mass of silver-109 is 108.9048 amu. Use the atomic mass of silver to calculate the relative abundance of silver-107. Show your xalculations. Answer 3. Complete the following conversions. Show your work for each. a. 214 km/s b. 643 mk/s cm/s Answer HK/ms Answer: 4. The following neutral elements form ions. What is the expected charge for each ion? a. F Answer: b. U Answer: C. Mg Answer: d. S Answer: 5. For the reaction below, calculate the mass (in grams) of the product formed when 15.54 g of the underlined reactant completely reacts. Assume that there is more than enough of the other reactant. Show your calculations. 2K (1) Cl g) 2 KC (s) 6. Copper (1) fluoride contains 37.42% F by mass. Use this percentage to calculate the mass of fluorine contained in 58.0 g of copper (1) fluoride. Show your calculations. Answer: 7. Enter a formula for the compound that forms between beryllium and each polyatomic ion. a. hydroxide Answer: b. chromate Answer: c. phosphate Answer:
Step by Step Solution
There are 3 Steps involved in it
Step: 1
1 A metal cube has an edge of 160mm and a mass of 367g Calculate the density of the metal Solution 1 Identify the data and what to find Edge length of the cube s 160 mm convert to meters later Mass of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


