Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A more detailed set of reactions describing the Chapman mechanism are as follows: (1) (2) O2+hv0+0 O3+hv O2 + O(3P) k =6.0 x
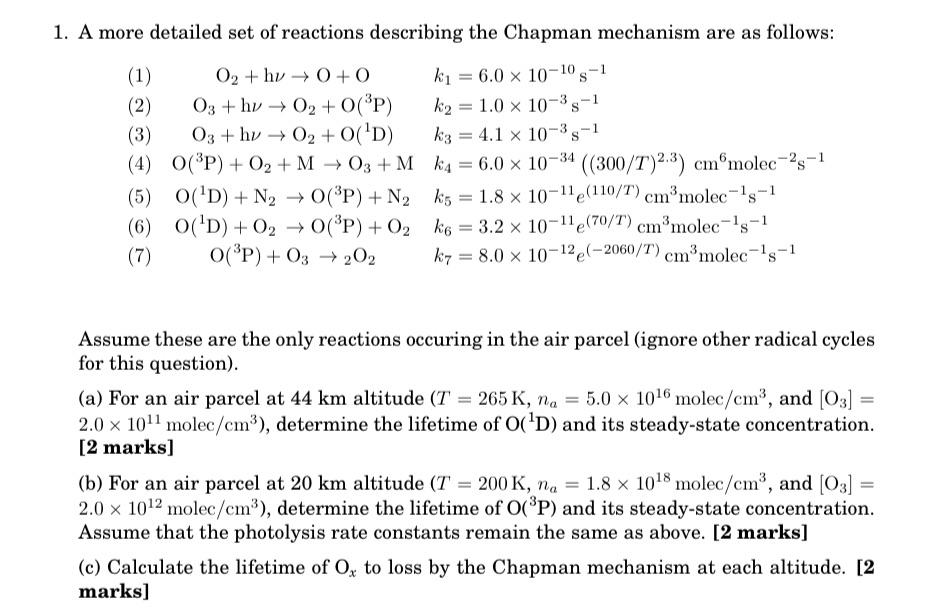
1. A more detailed set of reactions describing the Chapman mechanism are as follows: (1) (2) O2+hv0+0 O3+hv O2 + O(3P) k =6.0 x 10-10-1 k2 = 1.0 x 10-3-1 03+hv02+0('D) k3 4.1 x 10-3 s-1 (3) (4) O(3P) + O2 + MO3+Mk4 = 6.0 x 10-34 ((300/T) 2.3) cm molec-28-1 (5) O(D)+N2O(3P) + N2 (6) O(D)+020(3P) + O2 (7) O(3P) + 03 202 k5 = 1.8 10-11 (110/T) cm molec-18-1 e kg = 3.2 10-11 (70/T) cm molec-1s-1 k7 8.0 x 10-12 (-2060/T) cmmolec-1s-1. = Assume these are the only reactions occuring in the air parcel (ignore other radical cycles for this question). (a) For an air parcel at 44 km altitude (T = 265 K, na = 5.0 1016 molec/cm, and [03] 2.0 x10 molec/cm), determine the lifetime of O('D) and its steady-state concentration. [2 marks] (b) For an air parcel at 20 km altitude (T = 200 K, na = 1.8 1018 molec/cm, and [03] 2.0 x 1012 molec/cm), determine the lifetime of O(3P) and its steady-state concentration. Assume that the photolysis rate constants remain the same as above. [2 marks] (c) Calculate the lifetime of Ox to loss by the Chapman mechanism at each altitude. [2 marks]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


