Question
1 (a) Most hydrocarbons present in fuels can be classified as either paraffins, olefins, naphthenes, or aromatics. For each type of hydrocarbon, describe the identifying
1 (a) Most hydrocarbons present in fuels can be classified as either paraffins, olefins, naphthenes, or aromatics. For each type of hydrocarbon, describe the identifying features of the chemical structure (in terms of single bonds and double bonds for example), provide the chemical formula of an example, and comment on the H/C ratio.
(b) Define vapour pressure, lean flammability limit, and flashpoint, then explain how they are related using a plot of vapour pressure against temperature.
(c) Using the reference data provided below, show that the stoichiometric air-fuel ratio by mass for a hydrocarbon with an H/C ratio of y is given by: 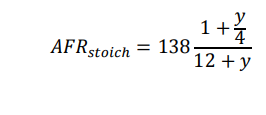
Reference data: Relative atomic mass: H: 1, C: 12, N: 14, O: 16 Composition of air: 20.9% O2, 79.1% N2 (vol%)
AFRstoich=13812+y1+4yStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


