Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A non-catalytic first order heterogeneous reaction involving a solid and a gaseous reactant is occurring in a 0.6m diameter and 9m high up-flow packed
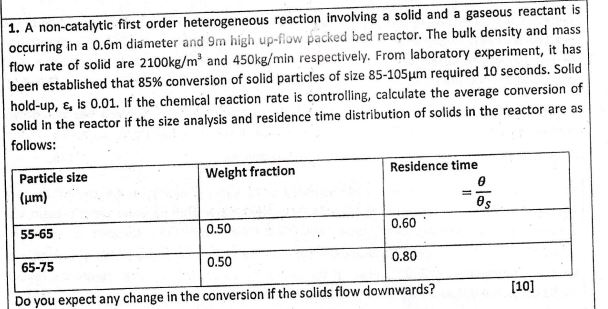 1. A non-catalytic first order heterogeneous reaction involving a solid and a gaseous reactant is occurring in a 0.6m diameter and 9m high up-flow packed bed reactor. The bulk density and mass flow rate of solid are 2100kg/m3 and 450kg/min respectively. From laboratory experiment, it has been established that 85% conversion of solid particles of size 85105m required 10 seconds. Solid hold-up, 3 is 0.01 . If the chemical reaction rate is controlling, calculate the average conversion of solid in the reactor if the size analysis and residence time distribution of solids in the reactor are as follows
1. A non-catalytic first order heterogeneous reaction involving a solid and a gaseous reactant is occurring in a 0.6m diameter and 9m high up-flow packed bed reactor. The bulk density and mass flow rate of solid are 2100kg/m3 and 450kg/min respectively. From laboratory experiment, it has been established that 85% conversion of solid particles of size 85105m required 10 seconds. Solid hold-up, 3 is 0.01 . If the chemical reaction rate is controlling, calculate the average conversion of solid in the reactor if the size analysis and residence time distribution of solids in the reactor are as follows Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


