Question
1. A standard addition experiment was done to determine the concentration of calcium, Ca in wastewater samples by using flame atomic absorption spectrometer (FAAS). 10
1. A standard addition experiment was done to determine the concentration of calcium, Ca in wastewater samples by using flame atomic absorption spectrometer (FAAS). 10 mL of aliquots were each added into 50 mL volumetric flasks. The response of the aliquots were measured and the following data were obtained.
| Solution | Concentration of added Ca2+ standard (ppb) | Absorbance |
| Blank | 0.0 | 0.018 |
| Addition 1 | 12.5 | 0.094 |
| Addition 2 | 25.0 | 0.139 |
| Addition 3 | 37.5 | 0.179 |
a. Sketch and label the standard addition graph.
b. Determine the concentration of Ca in the wastewater if the value of x-intercept from the graph, (CSA) is -7.0 ppb
c. The analysis of calcium can also be performed with flame atomic emission spectrometer (FAES). Differentiate between any two (2) principles in FAAS and FAES.
2. Given the following data:
| Compound | Retention time (min) |
| Unretained species | 0.87 |
| Methyl paraben | 3.23 |
| Propyl paraben | 5.16 |
a) Calculate the capacity factor, k' for methyl paraben and propyl paraben.
b) Calculate the selectivity factor, a for the two-component mixture.
3. A sample of mixture consisting of several cooking oils is subjected to gas chromatography analysis.
| Type of oils | Smoke point CC) |
| Flax seed | 107 |
| Peanut | 160 |
| Almond | 216 |
| Avocado | 271 |
a) Construct a temperature programming method of this analysis.
b) Suggest the most suitable detector for this analysis.
c) Briefly justify your answer in (b).
4. a) Briefly explain split and splitless injection.
b) State any two (2) criteria of a carrier gas and give any one (1) example.
5. a. Suggest a stationary phase-mobile phase combination of a normal phase HPLC.
b. Arrange the eluting order of n-hexane, 2-hexanol and pentanoic acid using a nonpolar column (Cl 8).
c. Briefly justify your answer in (b).
6. A student is running an HPLC sugar analysis using a C18 bonded column in a 1:4 water:methanol solvent system. He found two peaks in his chromatogram, D-fructose (labeled as 1) and D-glucose ( labelled as 2).
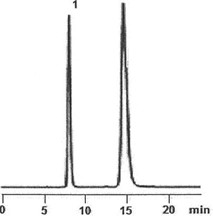
a) Based on the chromatogram, conclude which sugar is more polar.
b) Suggest a method to decrease the elution time of these sugars.
c) Justify your answer in (b).
0 5 10 15 20 minStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


