Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A student determines the freezing point of a solution of 0.92 g of unknown in 23.78 g of t-butyl alcohol. He obtains the
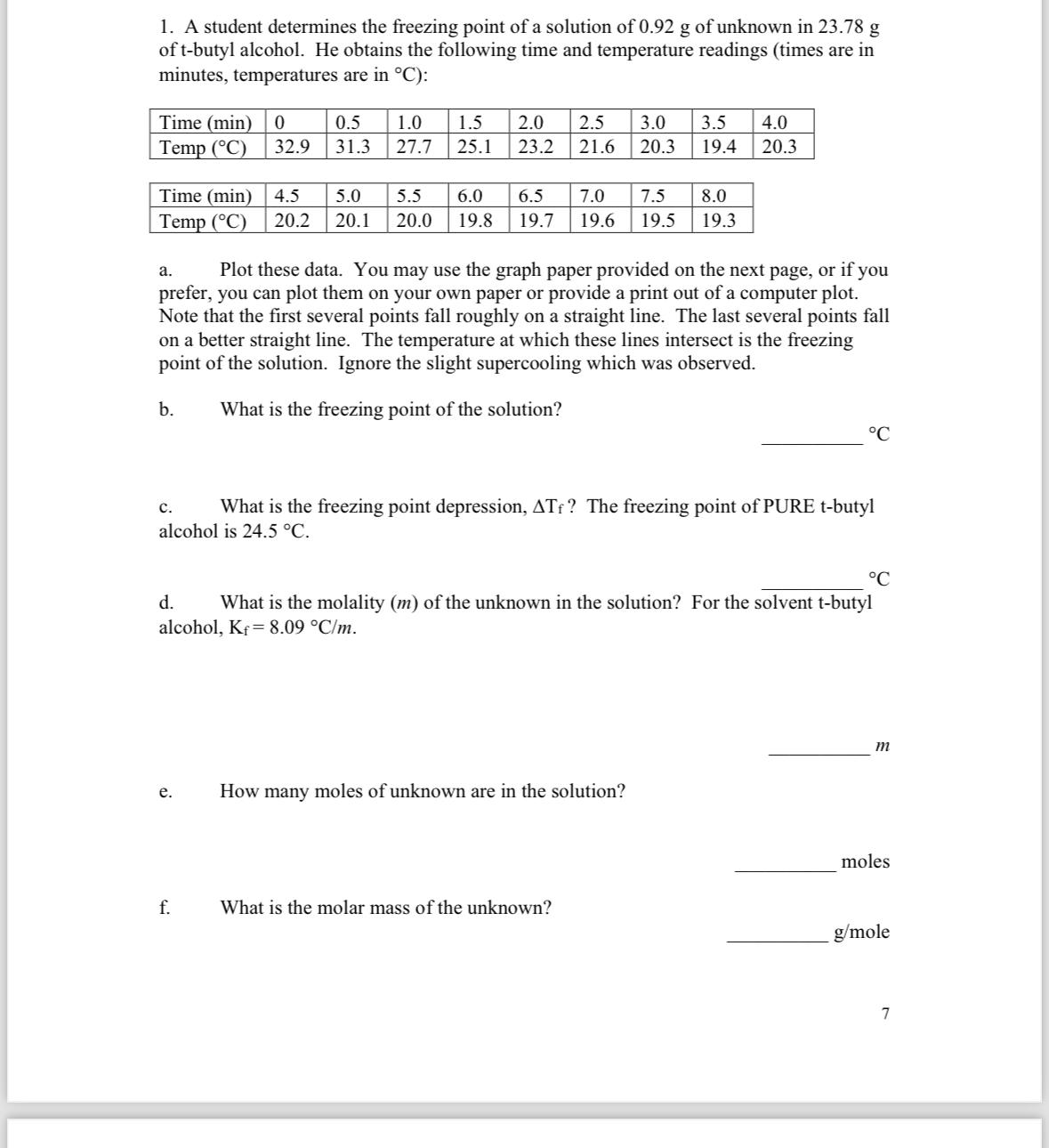
1. A student determines the freezing point of a solution of 0.92 g of unknown in 23.78 g of t-butyl alcohol. He obtains the following time and temperature readings (times are in minutes, temperatures are in C): Time (min) 0 Temp (C) Time (min) 5.0 4.5 Temp (C) 20.2 20.1 32.9 C. 0.5 31.3 e. 1.0 27.7 a. Plot these data. You may use the graph paper provided on the next page, or if you prefer, you can plot them on your own paper or provide a print out of a computer plot. Note that the first several points fall roughly on a straight line. The last several points fall on a better straight line. The temperature at which these lines intersect is the freezing point of the solution. Ignore the slight supercooling which was observed. b. What is the freezing point of the solution? f. 1.5 2.0 2.5 3.0 3.5 4.0 25.1 23.2 21.6 20.3 19.4 20.3 What is the freezing point depression, ATf? The freezing point of PURE t-butyl alcohol is 24.5 C. 5.5 6.0 6.5 7.0 7.5 8.0 20.0 19.8 19.7 19.6 19.5 19.3 d. What is the molality (m) of the unknown in the solution? For the solvent t-butyl alcohol, Kf = 8.09 C/m. How many moles of unknown are in the solution? C What is the molar mass of the unknown? m moles g/mole 7
Step by Step Solution
★★★★★
3.51 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
The image contains a laboratory assignment data table and several questions regarding the determination of the freezing point of a solution containing ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


