Question
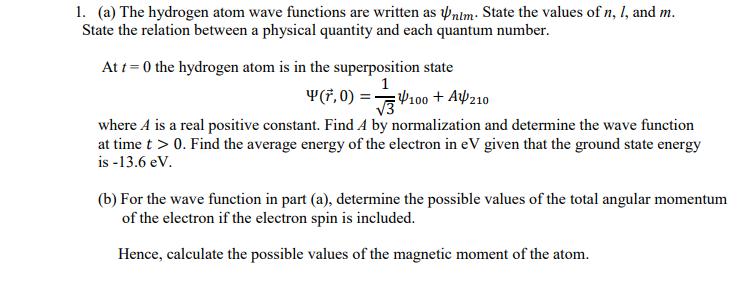
1. (a) The hydrogen atom wave functions are written as Pnim: State the values of n, I, and m. State the relation between a
1. (a) The hydrogen atom wave functions are written as Pnim: State the values of n, I, and m. State the relation between a physical quantity and each quantum number. At t = 0 the hydrogen atom is in the superposition state 1 Y(F, 0) = 100 + Af210 V3 where A is a real positive constant. Find A by normalization and determine the wave function at time t > 0. Find the average energy of the electron in eV given that the ground state energy is -13.6 eV. (b) For the wave function in part (a), determine the possible values of the total angular momentum of the electron if the electron spin is included. Hence, calculate the possible values of the magnetic moment of the atom.
Step by Step Solution
3.41 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantum Chemistry
Authors: Ira N. Levine
7th edition
321803450, 978-0321803450
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App