Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1- Alkanes are characterized by the general molecular formula: A. CnH2n-2 B. CnH2n C. CnH2n+2 2-Cycloalkanes are characterized by the general molecular formula: A.



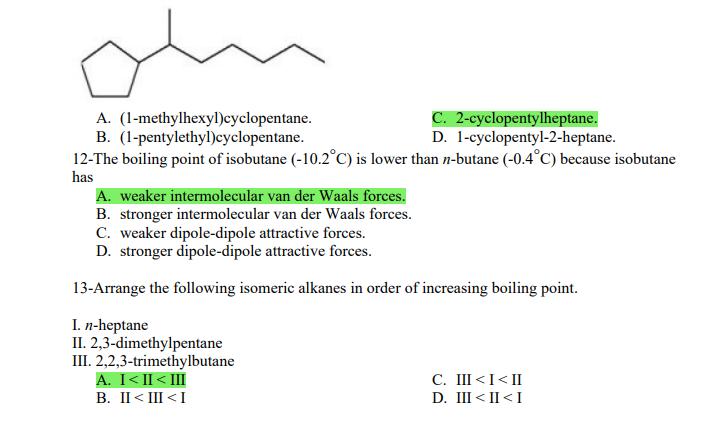
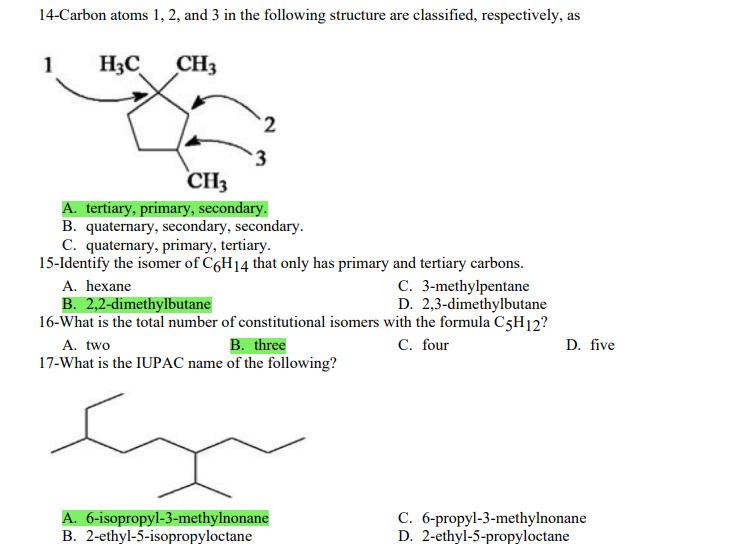
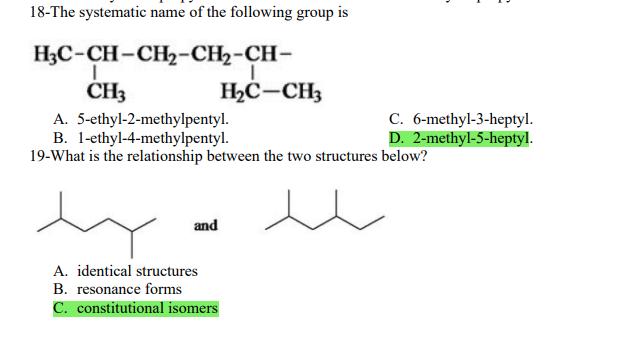

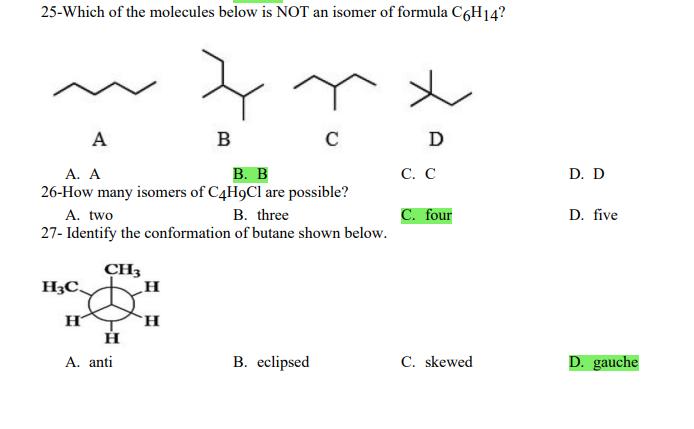
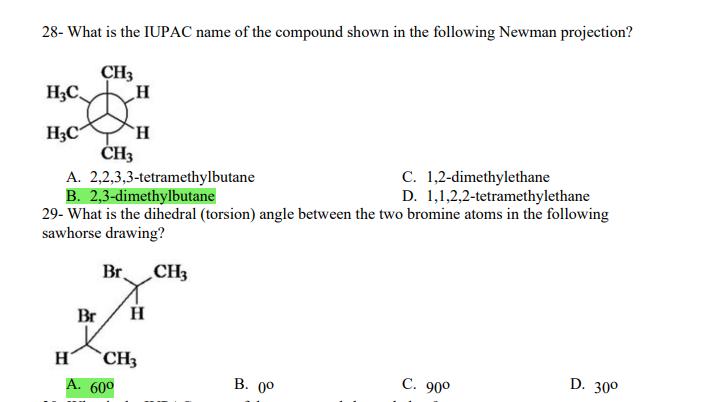
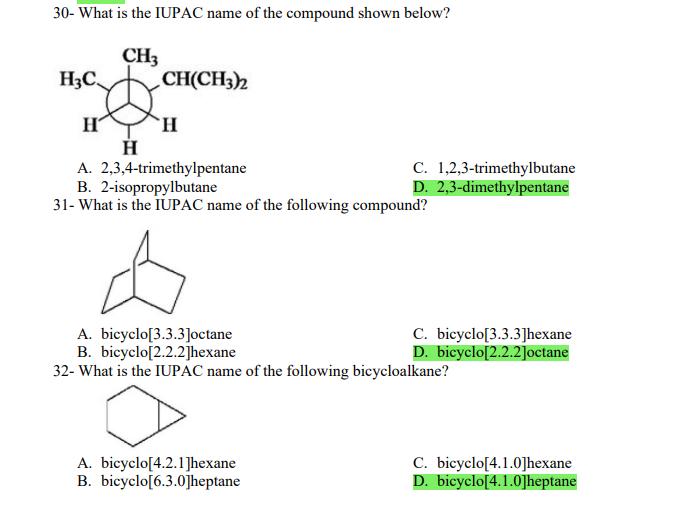

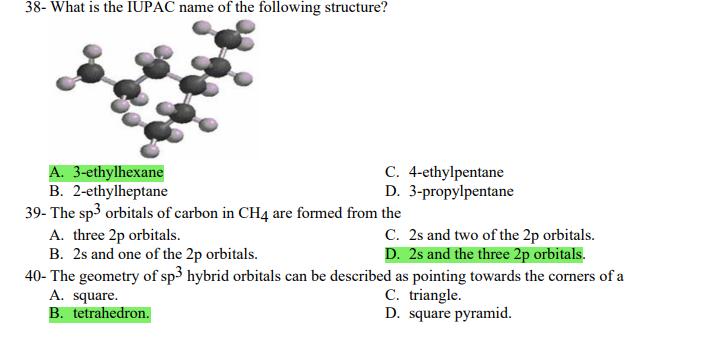
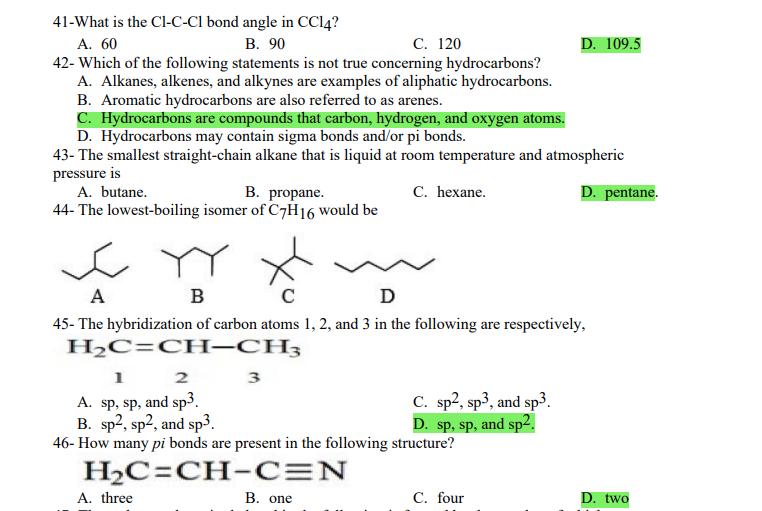

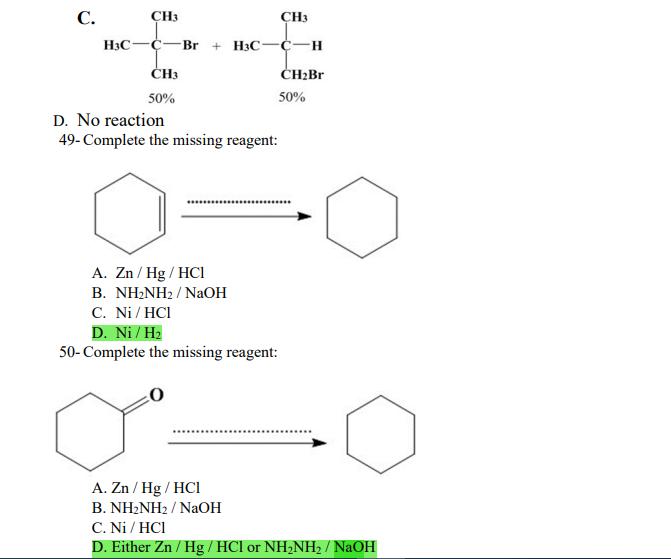
1- Alkanes are characterized by the general molecular formula: A. CnH2n-2 B. CnH2n C. CnH2n+2 2-Cycloalkanes are characterized by the general molecular formula: A. CnH2n-2 B. CnH2n C. CnH2n+2 3-What is the IUPAC name of the following compound? CH3 CH3-CH-CH-C-CH3 A. 4,4-dimethylpentane B. 1-tert-butylpropane 4-The common name of the following group is CH3CHCH- I CH3 CH3 A. n-butyl B. sec-butyl 5-Which one of the following is 2,2,5-trimethylhexane? A. (CH3)2CHCH2C(CH3)3 B. (CH3)2CHCHCHC(CH3)3 6-The correct IUPAC name of the following is CH3 HC-CH-CH2-CH-CH2-CH-CH-CH3 I CH3 I HC-CH3 A. 2,4,7-trimethylnonane. B. 7-ethyl-2,4-dimethyloctane. 7-What is the IUPAC name of the following? C. 2,2-dimethylpentane D. 1,1,1-trimethylbutane D. CnH2n+4 D. CnH2n+4 C. isobutyl C. CH3CH2CH(CH3)C(CH3)3 D. (CH3)2CHCH2 CH2CH2C(CH3)3 D. tert-butyl C. 3,6,8-trimethylnonane. D. 2-ethyl-5,7-dimethyloctane. 7-What is the IUPAC name of the following? CHCH3 CH3CHCHCHCHCHCH3 T CHCH3 A. 5,6-diethylheptane B. 5-ethyl-6-methyloctane C. 2,3-diethylheptane D. 4-ethyl-3-methyloctane 8-What is the IUPAC name of the following? A. 1-ethyl-4.4-dimethylcyclopentane B. 1-ethyl-3,3-dimethylcyclopentane 9-All the carbons in cyclopentane are A. primary carbons. B. secondary carbons. 10-The correct name of the following compound is C. 3-ethyl-1,1-dimethylcyclopentane D. 4-ethyl-1,1-dimethylcyclopentane C. tertiary carbons. D. quaternary carbons. A. (1-methylpropyl)cyclohexane. B. (2-methylpropyl)cyclohexane. 11-The correct IUPAC name of the following compound is C. (2,2-dimethylethyl)cyclohexane. D. (2,2-dimethylpropyl)cyclohexane. A. (1-methylhexyl)cyclopentane. C. 2-cyclopentylheptane. D. 1-cyclopentyl-2-heptane. B. (1-pentylethyl)cyclopentane. 12-The boiling point of isobutane (-10.2C) is lower than n-butane (-0.4C) because isobutane has A. weaker intermolecular van der Waals forces. B. stronger intermolecular van der Waals forces. C. weaker dipole-dipole attractive forces. D. stronger dipole-dipole attractive forces. 13-Arrange the following isomeric alkanes in order of increasing boiling point. I. n-heptane II. 2,3-dimethylpentane III. 2,2,3-trimethylbutane A. I < II < III B. II < III 14-Carbon atoms 1, 2, and 3 in the following structure are classified, respectively, as CH3 1 H3C 2 3 CH3 A. tertiary, primary, secondary. B. quaternary, secondary, secondary. C. quaternary, primary, tertiary. 15-Identify the isomer of C6H14 that only has primary and tertiary carbons. A. hexane B. 2,2-dimethylbutane 16-What is the total number of constitutional isomers with the formula C5H12? C. four A. two B. three 17-What is the IUPAC name of the following? A. 6-isopropyl-3-methylnonane B. 2-ethyl-5-isopropyloctane C. 3-methylpentane D. 2,3-dimethylbutane D. five C. 6-propyl-3-methylnonane D. 2-ethyl-5-propyloctane 18-The systematic name of the following group is H3C-CH-CH-CH-CH- I CH3 A. 5-ethyl-2-methylpentyl. B. 1-ethyl-4-methylpentyl. 19-What is the relationship between the two structures below? u and I HC-CH3 A. identical structures B. resonance forms C. constitutional isomers C. 6-methyl-3-heptyl. D. 2-methyl-5-heptyl. D. different compounds with different compositions 20-Arrange the following hydrocarbons in order of increasing boiling point. I. pentane II. 2,2-dimethylpropane III. 2-methylbutane A. I < II < III B. I < III < II 21-The 1,1-dimethylethyl group, -C(CH3)3, can also be called A. butyl. B. isobutyl. 22-What is the relationship between the following two structures? and C. II < I < III D. II < III 25-Which of the molecules below is NOT an isomer of formula C6H14? A H3C. A. A B. B 26-How many isomers of C4H9Cl are possible? H A. two B. three 27- Identify the conformation of butane shown below. CH3 H B A. anti H H C B. eclipsed x D C. C C. four C. skewed D. D D. five D. gauche 28- What is the IUPAC name of the compound shown in the following Newman projection? CH3 H HC H3C CH3 C. 1,2-dimethylethane D. 1,1,2,2-tetramethylethane 29- What is the dihedral (torsion) angle between the two bromine atoms in the following sawhorse drawing? Br CH3 H H A. 2,2,3,3-tetramethylbutane B. 2,3-dimethylbutane Br H CH3 A. 600 B. 00 C. 90 D. 30 30- What is the IUPAC name of the compound shown below? CH3 H3C CH(CH3)2 H H A. 2,3,4-trimethylpentane B. 2-isopropylbutane 31- What is the IUPAC name of the following compound? H C. 1,2,3-trimethylbutane D. 2,3-dimethylpentane A. bicyclo[4.2.1]hexane B. bicyclo[6.3.0]heptane A. bicyclo[3.3.3]octane B. bicyclo[2.2.2]hexane 32- What is the IUPAC name of the following bicycloalkane? C. bicyclo[3.3.3]hexane D. bicyclo[2.2.2 Joctane C. bicyclo[4.1.0]hexane D. bicyclo[4.1.0]heptane 33- Identify the relationship between the following two Newman projections. H HC H H CH3 H H CH3 H CH HC H and A. constitutional isomers B. stereoisomers 34- Which one of the following is the butane conformation shown below? HC HC A. anti B. eclipsed C. skewed 35- Which of the staggered conformations of 2-methylbutane is most stable? CH H CH; CH A. secondary carbons. B. quaternary carbons. CH3 CH In Hi H CH H A B 36- Cyclohexane is composed of A. methyl groups. B. methylene groups. 37- All the carbons in cyclopentane are H C. identical D. different conformations of the same compound HCH: D CH H D. gauche C. methine groups. D. both methine and methylene groups. C. primary carbons. D. tertiary carbons. 38- What is the IUPAC name of the following structure? A. 3-ethylhexane B. 2-ethylheptane C. 4-ethylpentane D. 3-propylpentane 39- The sp3 orbitals of carbon in CH4 are formed from the A. three 2p orbitals. B. 2s and one of the 2p orbitals. C. 2s and two of the 2p orbitals. D. 2s and the three 2p orbitals. 40- The geometry of sp3 hybrid orbitals can be described as pointing towards the corners of a A. square. C. triangle. B. tetrahedron. D. square pyramid. 41-What is the Cl-C-Cl bond angle in CC14? A. 60 B. 90 C. 120 42- Which of the following statements is not true concerning hydrocarbons? A. Alkanes, alkenes, and alkynes are examples of aliphatic hydrocarbons. B. Aromatic hydrocarbons are also referred to as arenes. C. Hydrocarbons are compounds that carbon, hydrogen, and oxygen atoms. D. Hydrocarbons may contain sigma bonds and/or pi bonds. 43- The smallest straight-chain alkane that is liquid at room temperature and atmospheric pressure is A. butane. C. hexane. D. pentane. B. propane. 44- The lowest-boiling isomer of C7H16 would be XYY X C A B D 45- The hybridization of carbon atoms 1, 2, and 3 in the following are respectively, H,C=CH-CH3 1 2 A. sp, sp, and sp3. B. sp2, sp2, and sp3. 3 A. three 46- How many pi bonds are present in the following structure? HC=CH-CEN C. sp2, sp3, and sp. D. sp, sp, and sp2. B. one D. 109.5 C. four D. two 47- The carbon-carbon single bond in the following is formed by the overlap of which two orbitals? HC=CH-C=N B. sp-sp3 A. sp-sp 48- Complete the following equation: CH3 A. B. H3C CH3 H3C -C-H CHBr CH3 CH3 H3C-C- Br CH3 H Br2/ hy C. sp2-sp2 D. sp-sp C. CH3 H3C C-Br+ H3C-C-H CH3 50% D. No reaction 49- Complete the missing reagent: CH3 CHBr 50% A. Zn/Hg/HCI B. NH,NHz / NaOH A. Zn/Hg/HCI B. NH2NH2/NaOH C. Ni/HCI D. Ni/H 50- Complete the missing reagent: **** C. Ni/HCI D. Either Zn/Hg/HCl or NHNH/NaOH
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 1 attachment)
63649e83350d5_106835.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


