Question
1. All chromatography processes separate sample components on the basis of partitioning between a stationary solid phase and a liquid mobile phase. One phase will
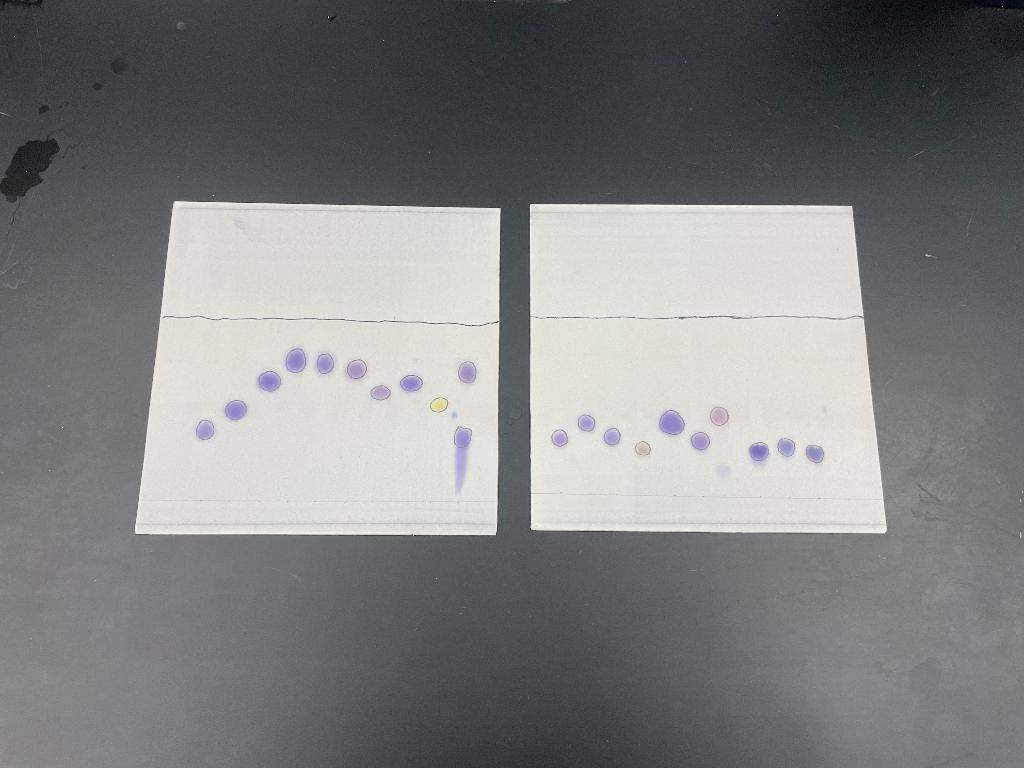
1. All chromatography processes separate sample components on the basis of partitioning between a stationary solid phase and a liquid mobile phase. One phase will be more hydrophilic (polar) and the other more hydrophobic (non-polar). Based on the relative movements of the amino acids in this chromatography system, which phase (mobile - solvent or stationary- TLC plate) is polar and which is non-polar. Explain your answer using Rf data from carefully selected representative hydrophobic, polar and basic amino acids.
2. Under the chromatographic conditions (amino acids dissolved in acid and the solvent contains acid), which of the amino acids have additional charges beyond those common to all of them? Remember that those R group pKas listed in table 5.2 mean that under acid conditions R-group carboxylic acid groups will be neutral (-COOH), while R-group amino groups will be positively charged (-NH3+). [Forget Cys and Tyr.] These charges explain why these 3 AAs move so poorly in the chromatography system that we used. How so?
3. GLY is listed as a hydrophobic amino acid. Why isnt it running with the others very well?
4. How do the structural differences between PHE and TYR explain their relative mobility differences?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


