Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.) Aqueous A at a concentration of 0.5 M is introduced into a batch reactor where it reacts away to form product R according
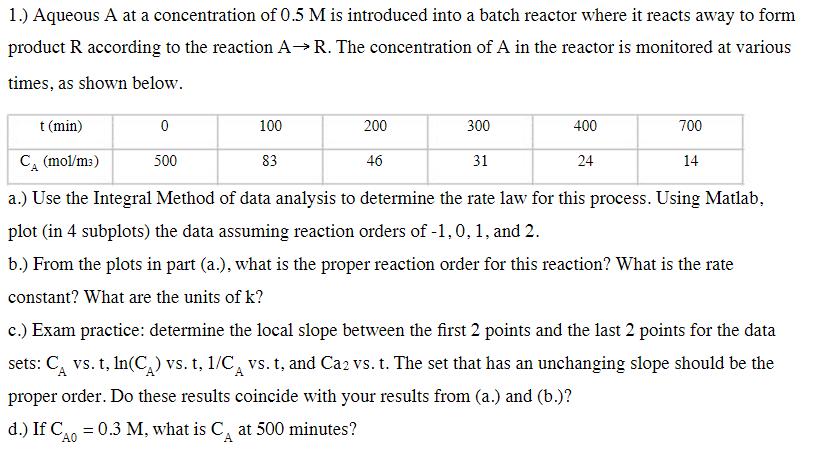
1.) Aqueous A at a concentration of 0.5 M is introduced into a batch reactor where it reacts away to form product R according to the reaction A R. The concentration of A in the reactor is monitored at various times, as shown below. t (min) CA (mol/m) 0 100 200 300 400 700 500 83 46 31 24 14 a.) Use the Integral Method of data analysis to determine the rate law for this process. Using Matlab, plot (in 4 subplots) the data assuming reaction orders of -1, 0, 1, and 2. b.) From the plots in part (a.), what is the proper reaction order for this reaction? What is the rate constant? What are the units of k? c.) Exam practice: determine the local slope between the first 2 points and the last 2 points for the data sets: C vs. t, ln(CA) vs. t, 1/C vs. t, and Ca2 vs. t. The set that has an unchanging slope should be the proper order. Do these results coincide with your results from (a.) and (b.)? d.) If C = 0.3 M, what is C at 500 minutes? A0
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Using the Integral Method The rate law for a batch reactor can be expressed as rA dCAdt kCAn Integ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


