1. As shown by point D in Fig 3.1, the volume of an ideal diatomic gas is 2.00L at standard condition (STP, T=273.15K, P=101.3kPa).
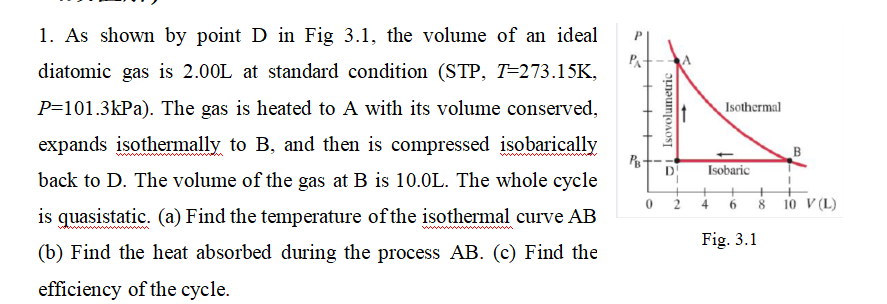
1. As shown by point D in Fig 3.1, the volume of an ideal diatomic gas is 2.00L at standard condition (STP, T=273.15K, P=101.3kPa). The gas is heated to A with its volume conserved, expands isothermally to B, and then is compressed isobarically back to D. The volume of the gas at B is 10.0L. The whole cycle is quasistatic. (a) Find the temperature of the isothermal curve AB (b) Find the heat absorbed during the process AB. (c) Find the efficiency of the cycle. PA- PB Isovolumetric D 0 2 Isothermal Isobaric 4 6 Fig. 3.1 10 V (L)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


