Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Below is molecule 1. Provide the relationship between the indicated protons below, either diastereotopic or identical and then answer the remaining questions about
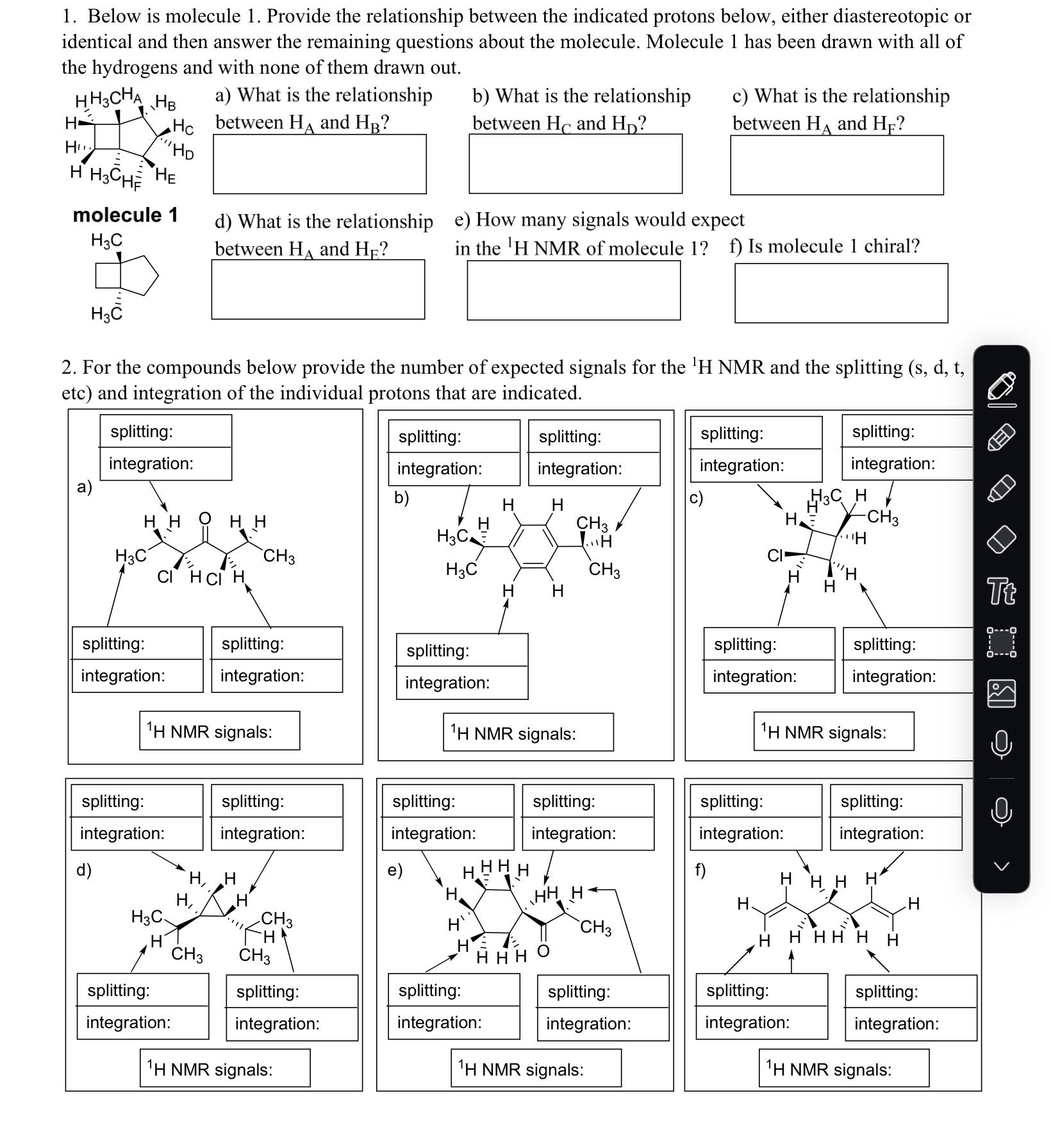
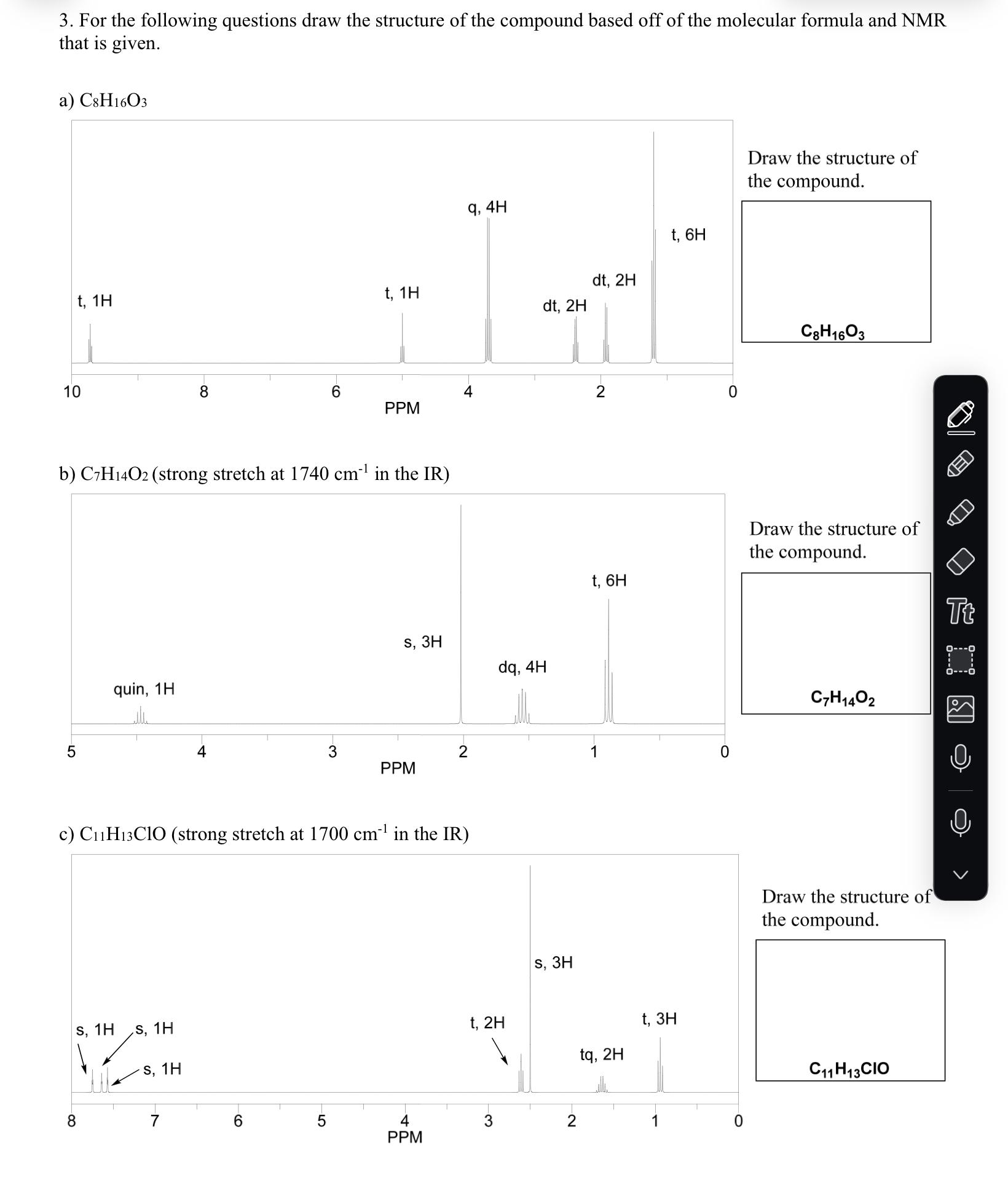
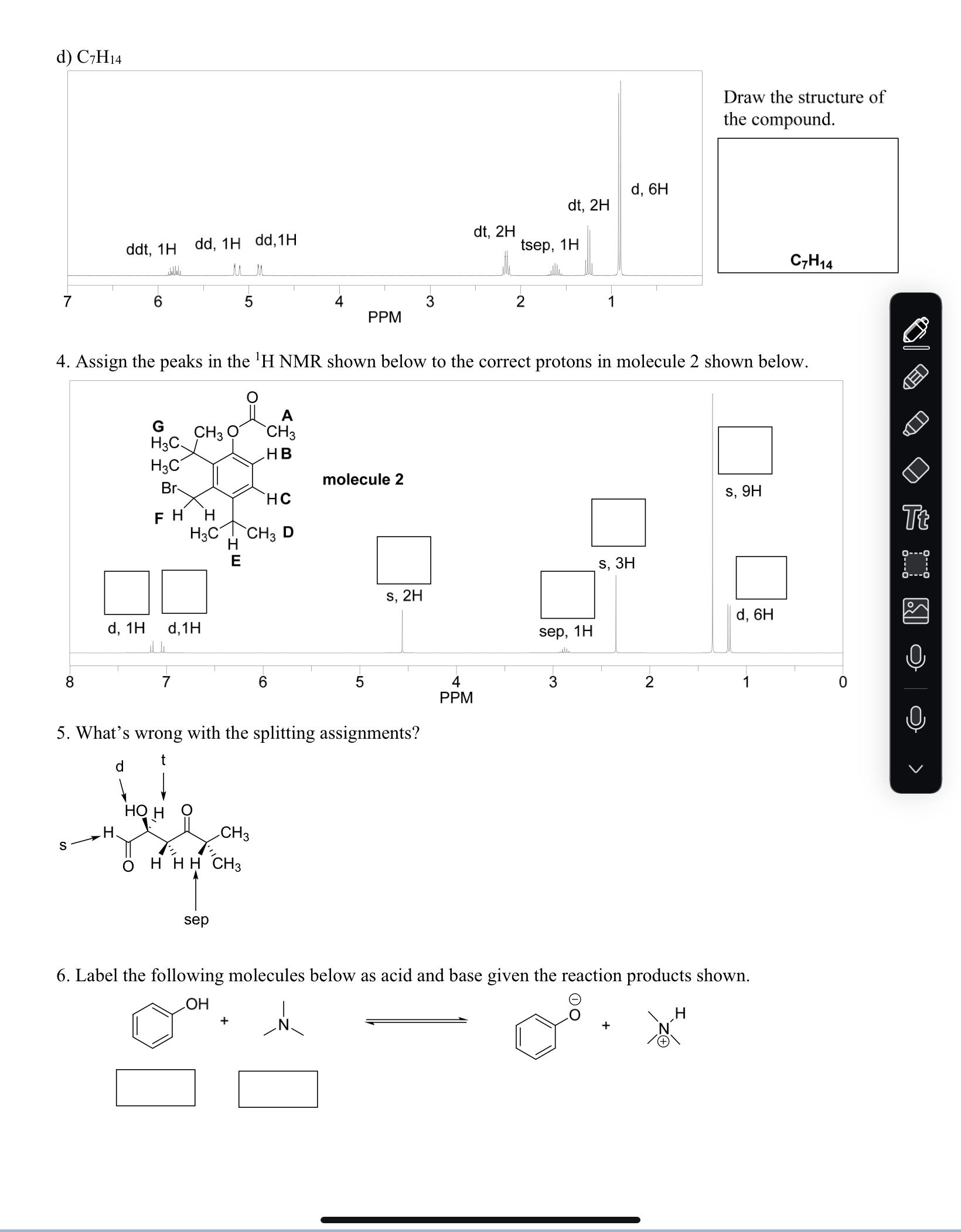
1. Below is molecule 1. Provide the relationship between the indicated protons below, either diastereotopic or identical and then answer the remaining questions about the molecule. Molecule 1 has been drawn with all of the hydrogens and with none of them drawn out. HH3CHA HB a) What is the relationship between HA and HB? b) What is the relationship c) What is the relationship H H HC HD between H and HD? between HA and HF? HH3CHF HE molecule 1 H3C d) What is the relationship between HA and HE? e) How many signals would expect in the 'H NMR of molecule 1? f) Is molecule 1 chiral? H 2. For the compounds below provide the number of expected signals for the 'H NMR and the splitting (s, d, t, etc) and integration of the individual protons that are indicated. splitting: integration: a) HH OHH H3C CH3 CI HCI H splitting: integration: splitting: integration: splitting: integration: b) H H H3C CH3 H H3C CH3 H H splitting: integration: H3CH H -CH3 H Tt splitting: splitting: splitting: integration: integration: splitting: integration: splitting: integration: integration: 1H NMR signals: 1H NMR signals: 1H NMR signals: splitting: splitting: splitting: splitting: splitting: integration: integration: integration: integration: integration: splitting: integration: d) e) f) H, H HHHH H HHH H, H H H H3C H CH3 CH3 H HHHHH CH3 CH3 HHHO splitting: integration: splitting: splitting: integration: integration: splitting: integration: splitting: integration: 1H NMR signals: 1H NMR signals: splitting: integration: 1H NMR signals: 3. For the following questions draw the structure of the compound based off of the molecular formula and NMR that is given. a) C8H16O3 t, 1H 9, 4H t, 6H t, 1H dt, 2H dt, 2H 10 8 6 4 2 PPM b) C7H14O2 (strong stretch at 1740 cm in the IR) 5 quin, 1H S, 3H dq, 4H t, 6H 3 2 1 0 PPM c) C11H13CIO (strong stretch at 1700 cm in the IR) 8 S, 1H S, 1H S, 1H t, 2H S, 3H 6 5 4 3 2 PPM tq, 2H t, 3H Draw the structure of the compound. C8H1603 Draw the structure of the compound. C7H14O2 Draw the structure of the compound. C11 H13CIO Tt d) C7H14 ddt, 1H dd, 1H dd, 1H d, 6H dt, 2H dt, 2H tsep, 1H 7 6 5 4 3 2 1 PPM Draw the structure of the compound. C7H14 4. Assign the peaks in the 'H NMR shown below to the correct protons in molecule 2 shown below. G CH3 A CH3 H3C HB H3C Br molecule 2 HC FH H H3C CH3 D E d, 1H d, 1H 8 7 6 5 S, 2H 5. What's wrong with the splitting assignments? d HO H H CH3 S HHH CH3 sep sep, 4 3 PPM 1H S, 3H S, 9H Tt d, 6H 2 1 0 6. Label the following molecules below as acid and base given the reaction products shown. OH + +
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


