Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Calculate the mass of a molecule of N2O3. The atomic masses of N and O are 14.01 amu/atom and 16.00 amu/atom respectively. 2.
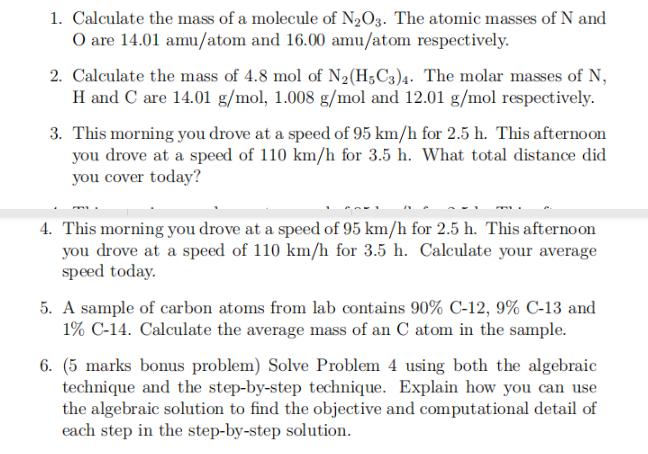

1. Calculate the mass of a molecule of N2O3. The atomic masses of N and O are 14.01 amu/atom and 16.00 amu/atom respectively. 2. Calculate the mass of 4.8 mol of N2(H5C3)4. The molar masses of N, H and C are 14.01 g/mol, 1.008 g/mol and 12.01 g/mol respectively. 3. This morning you drove at a speed of 95 km/h for 2.5 h. This afternoon you drove at a speed of 110 km/h for 3.5 h. What total distance did you cover today? 4. This morning you drove at a speed of 95 km/h for 2.5 h. This afternoon you drove at a speed of 110 km/h for 3.5 h. Calculate your average speed today. 5. A sample of carbon atoms from lab contains 90% C-12, 9% C-13 and 1% C-14. Calculate the average mass of an C atom in the sample. 6. (5 marks bonus problem) Solve Problem 4 using both the algebraic technique and the step-by-step technique. Explain how you can use the algebraic solution to find the objective and computational detail of each step in the step-by-step solution. For each of the problems 1 to 5 (inclusive) a. (1 mark) List the unknown quantity name, unknown quantity symbol, unknown unit name and unknown unit symbol. b. (1 mark) Write an equation that models the problem. c. (8 mark) Solve the problem using either the algebraic technique or the step-by-step technique (not both).
Step by Step Solution
★★★★★
3.59 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Lets calculate the correct answers to the given questions 1 Mass of a molecule of N2O3 Atomic masses ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


