Table 6.6-1 The Critical and Other Constants for Selected Fluids Molecular Weight (g mol-) V. (m/kmol) Substance Symbol T.(K) P (MPa) Z. Toil (K)
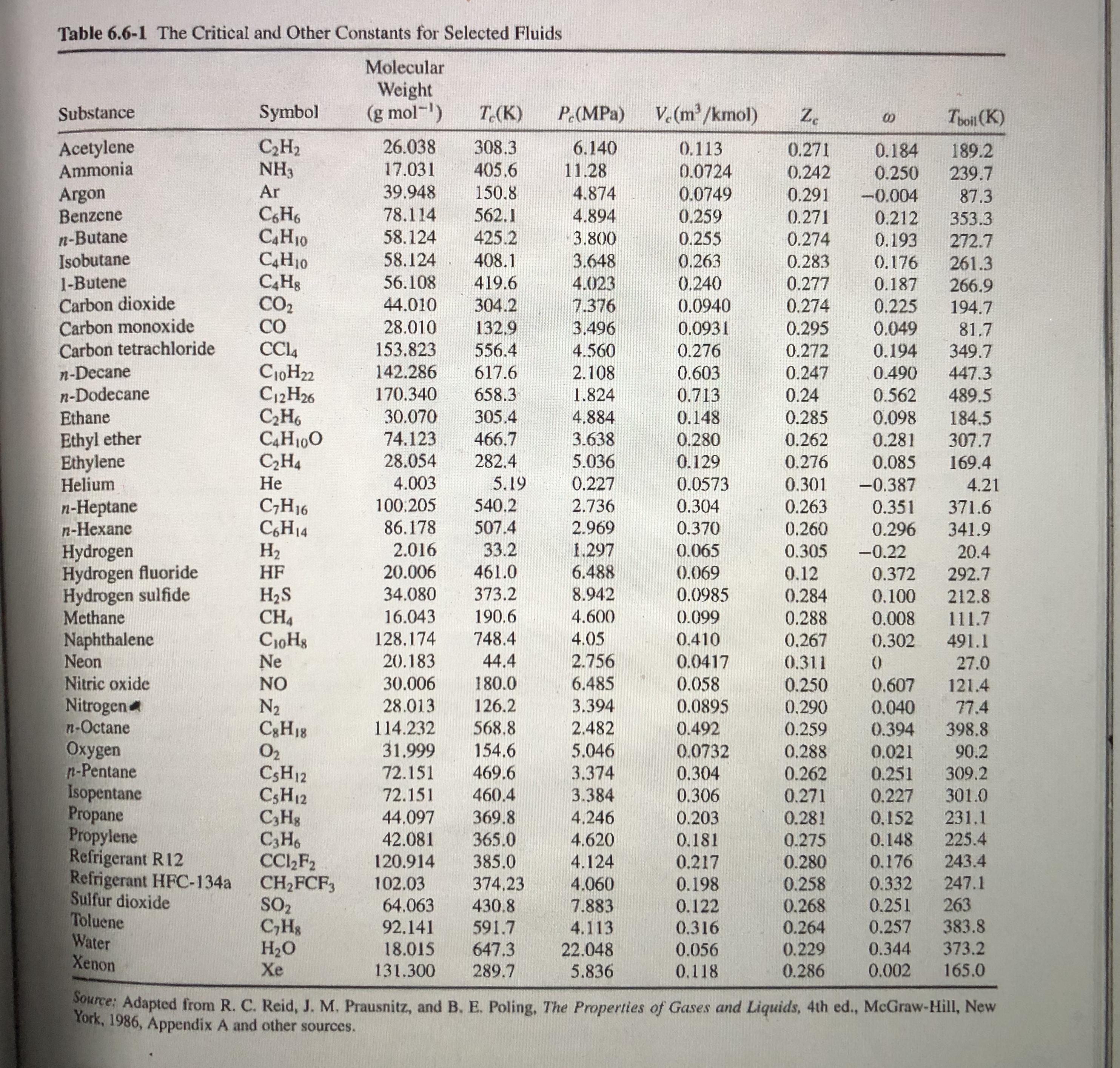

Table 6.6-1 The Critical and Other Constants for Selected Fluids Molecular Weight (g mol-) V. (m/kmol) Substance Symbol T.(K) P (MPa) Z. Toil (K) Acetylene Ammonia C,H2 NH3 26.038 308.3 405.6 6.140 0.113 0.271 0.184 0.250 189.2 17.031 11.28 0.0724 0.0749 0.242 239.7 Ar 39.948 150.8 Argon Benzene n-Butane Isobutane 1-Butene Carbon dioxide Carbon monoxide Carbon tetrachloride 4.874 0.291 -0.004 87.3 CH6 C4H10 C4H10 78,114 562.1 4.894 0.259 0.271 0.212 353.3 58.124 58.124 425.2 3.800 0.255 0.274 0.193 272.7 408.1 419.6 3.648 0.263 0.283 0.176 261.3 0.240 0.0940 56.108 4.023 0.277 0.187 266.9 194.7 CO2 CO 44.010 304.2 7.376 0.274 0.225 28.010 132,9 3.496 0.0931 0.295 0.049 81.7 349.7 153.823 556.4 4.560 0.276 0.272 0.194 n-Decane n-Dodecane Ethane C10H22 C12H26 C,H6 C4H100 C,H4 142.286 617.6 2.108 0.603 0.247 0.490 447.3 170.340 658.3 1.824 0.713 0.24 0.562 489.5 30.070 305.4 4.884 0.148 0.285 0.098 184.5 307.7 Ethyl ether Ethylene Helium 74.123 466.7 3.638 5.036 0.280 0.262 0.281 28.054 282.4 0.129 0.276 0.085 169.4 4.003 5.19 0.227 0.0573 0.301 -0.387 4.21 540.2 n-Heptane n-Hexane C;H16 C,H14 H2 100.205 2.736 0.304 0.263 0.351 371.6 86.178 507.4 2.969 0.370 0.260 0.296 341.9 2.016 33.2 1.297 Hydrogen Hydrogen fluoride Hydrogen sulfide Methane Naphthalene Neon Nitric oxide Nitrogen n-Octane Oxygen n-Pentane Isopentane Propane Propylene Refrigerant R12 Refrigerant HFC-134a Sulfur dioxide Toluene Water Xenon 0.065 0.305 -0.22 20.4 | HF 20.006 461.0 6.488 (0.069 0.12 0.372 292.7 373.2 H2S CH4 CioHg Ne 34.080 8.942 0.0985 0.284 0.100 212.8 16.043 190.6 4.600 0.099 0.288 0.008 111.7 128,174 748.4 4.05 0.410 0.267 0.302 491.1 20.183 44.4 2.756 0.0417 0.058 0.0895 0.311 27.0 NO 30.006 180.0 6.485 0.250 0.607 121.4 N2 C3H18 O2 CSH12 CSH12 C,Hg C3H6 CC,F2 CH2FCF, SO2 C,Hs H2O Xe 28.013 126.2 3.394 0.290 0.040 77.4 114.232 568.8 2.482 0.492 0.259 0.288 0.394 398.8 31.999 154.6 5.046 0.0732 0.021 90.2 309.2 72.151 469.6 3.374 0.304 0.262 0.271 0.251 72.151 3.384 4,246 301.0 231.1 460.4 0.306 0.227 44.097 42.081 369.8 365.0 0.203 0.281 0,152 4.620 0.181 0.275 0.148 225.4 4.124 4.060 120.914 385.0 0.217 243.4 0.280 0.258 0.268 0.264 0.176 102.03 64.063 0.332 0.251 374,23 0.198 247.1 0.122 0.316 263 383.8 373.2 430.8 7.883 92.141 591.7 4.113 0.257 647.3 18.015 131.300 22.048 5.836 0.056 0.229 0.344 289.7 0.118 0.286 0.002 165.0 Source: Adapted from R. C. Reid, J. M. Prausnitz, and B. E. Poling, The Properties of Gases and Liquids, 4th ed., McGraw-Hill, New York, 1986, Appendix A and other sources.
Step by Step Solution
3.53 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


