Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Carbon dioxide with pressure 10 bar(g) and temperature 150 C flowarte is 100 kg/second flow Through duct with diameter 3 centimeters. Find stagnation
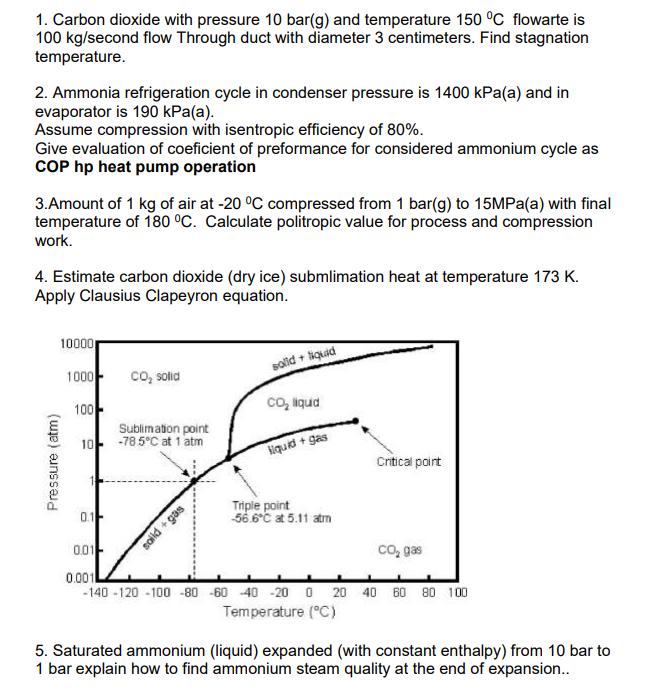
1. Carbon dioxide with pressure 10 bar(g) and temperature 150 C flowarte is 100 kg/second flow Through duct with diameter 3 centimeters. Find stagnation temperature. 2. Ammonia refrigeration cycle in condenser pressure is 1400 kPa(a) and in evaporator is 190 kPa(a). Assume compression with isentropic efficiency of 80%. Give evaluation of coeficient of preformance for considered ammonium cycle as COP hp heat pump operation 3.Amount of 1 kg of air at -20 C compressed from 1 bar(g) to 15MPa(a) with final temperature of 180 C. Calculate politropic value for process and compression work. 4. Estimate carbon dioxide (dry ice) submlimation heat at temperature 173 K. Apply Clausius Clapeyron equation. Pressure (atm) 10000 1000- CO solid solid + Siquid 100 CO liquid 10 Sublimation point -785C at 1 atm liquid + gas Critical point 0.1- 0.01 0.001 soild +gas Triple point 56.6C at 5.11 atm CO gas -140-120-100-80 -60 40 -20 0 20 40 60 80 100 Temperature (C) 5. Saturated ammonium (liquid) expanded (with constant enthalpy) from 10 bar to 1 bar explain how to find ammonium steam quality at the end of expansion..
Step by Step Solution
★★★★★
3.55 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Step1 Solution The question states that the carbon dioxide is at a pressure of 10 barg and a temperature of 150 C with a flow rate of 100 kgsecond thr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


