Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(1) Carry out energy balance for both conditions. Calculate the heat loss. (2) Calculate the theoretical (or stoichiometric) amount of oxygen required to fully combust
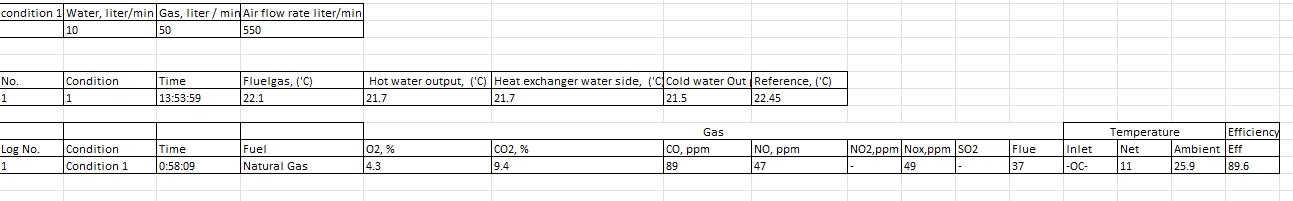
(1) Carry out energy balance for both conditions. Calculate the heat loss.
(2) Calculate the theoretical (or stoichiometric) amount of oxygen required to fully combust the gas for each steady state. Calculate the equivalence ratio () of your gas-air mixture.
(3) Calculate quantity of combustion by-products (mass of emissions and moisture).
\begin{tabular}{|l|l|l|l|} \hline condition 1 & Water, liter/min & Gas, liter / min & Air flow rate liter/min \\ \hline & 10 & 50 & 550 \\ \hline \end{tabular} \begin{tabular}{|l|l|l|l|l|l|l|l|l|} \hline No. & Condition & Time & Fluelgas, ('C) & Hot water output, ('C) & Heat exchanger water side, ('C) & Cold water Out & Reference, ('C) \\ \hline 1 & 1 & 13:53:59 & 22.1 & 21.7 & 21.7 & 21.5 \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


