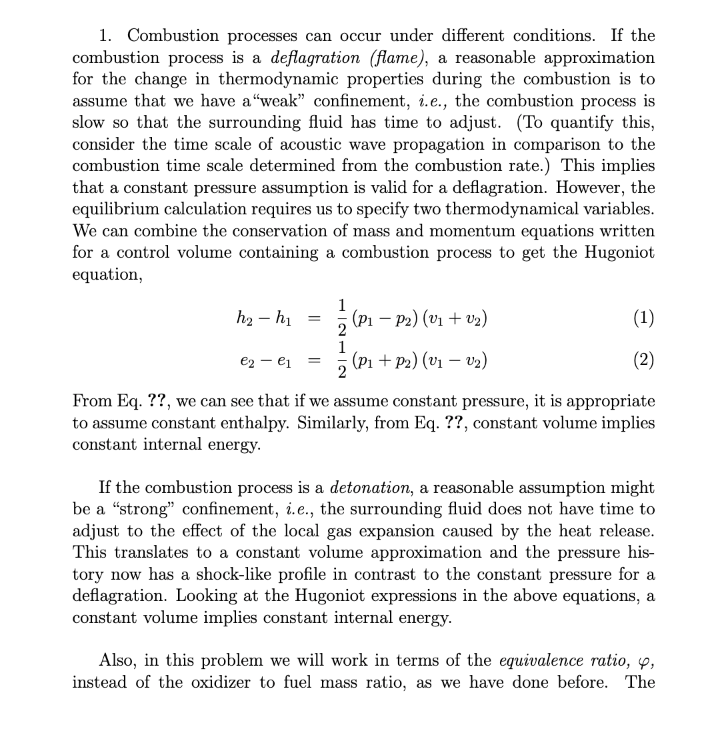


1. Combustion processes can occur under different conditions. If the combustion process is a deflagration (flame), a reasonable approximation for the change in thermodynamic properties during the combustion is to assume that we have a "weak confinement, i.e., the combustion process is slow so that the surrounding fluid has time to adjust. (To quantify this, consider the time scale of acoustic wave propagation in comparison to the combustion time scale determined from the combustion rate.) This implies that a constant pressure assumption is valid for a deflagration. However, the equilibrium calculation requires us to specify two thermodynamical variables. We can combine the conservation of mass and momentum equations written for a control volume containing a combustion process to get the Hugoniot equation, h2 hi (P1-P2) (V1 + 02) - (1) e2 - ei 3 (P1 + P2) (v1 12) (2) From Eq. ??, we can see that if we assume constant pressure, it is appropriate to assume constant enthalpy. Similarly, from Eq. ??, constant volume implies constant internal energy. If the combustion process is a detonation, a reasonable assumption might be a "strong" confinement, i.e., the surrounding fluid does not have time to adjust to the effect of the local gas expansion caused by the heat release. This translates to a constant volume approximation and the pressure his- tory now has a shock-like profile in contrast to the constant pressure for a deflagration. Looking at the Hugoniot expressions in the above equations, a constant volume implies constant internal energy. Also, in this problem we will work in terms of the equivalence ratio, 4, instead of the oxidizer to fuel mass ratio, as we have done before. The equivalence ratio has the interesting property that it is the same whether you work in terms of mass or moles, mfuel/mos Q= (m fuel/ nuelox (n fuelog) stoich mos) stoich where m and n are the mass in g/mole and the number of moles, respec- tively. The following mixture of gases are used as reactants in a combustion experiment: 2 mole of C2H2, 1 mole of 02, 2 mole of H2, and 6 mole of N, at an initial pressure 2.5 atm and an initial temperature 300 K. In terms of the equation above, consider both the species C,H, and H, to be the fuel and 02 to be the oxidizer. With this introductory material and the STANJAN code, do the following problems for the above mixture. Think about how you will run the code. (a) Calculate the equilibrium composition, temperature, and pressure of the combustion products after a deflagration. Additional possible reaction products include: CO2, H2O, H2, CO, OH, O, H, and C. (b) Solve for the stoichiometric value for the number of moles of the mixture constituents, analytically assuming that the products are CO2, H2O and N, and that the reactants are 2 moles of C2H2, 2 moles of H2, and 6 moles of N2. What is the stoichiometric f/o ratio? (c) What is the f/o ratio for the mixture in (a)? What is its equivalence ratio? Is it fuel or oxidizer rich? (d) Calculate the equilibrium composition, temperature, and pressure as a function of equivalence ratio by adding O2 to the original mixture (part a) until it reaches stoichiometric. Calculate six o conditions of 1,2,3,4,5,6. (Always use 2 moles of C2H2, 2 moles of H2, and 6 mole of N2, and vary the number of moles of O2 as required to obtain p.) Plot the results for temperature, pressure and for species with significant mole fractions (10-4) and higher). Plot P, and T each on separate graphs and all mole fractions on a single plot. (e) Assume that the combustion process proceeds through a detonation. Calculate the equilibrium composition, temperature, and pressure of the com- bustion products after the detonation for an equivalence ratio of 4. (f) How do your answers to part (d), equivalence ratio of 4, and part (e) change if you remove CO as a possible product









