Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Consider the following equilibrium: H2O(g)+CO(g)H2(g)+CO2(g) A closed container is initially filled with H2O and CO. As the reaction proceeds towards quilibrium: a) [CO] and


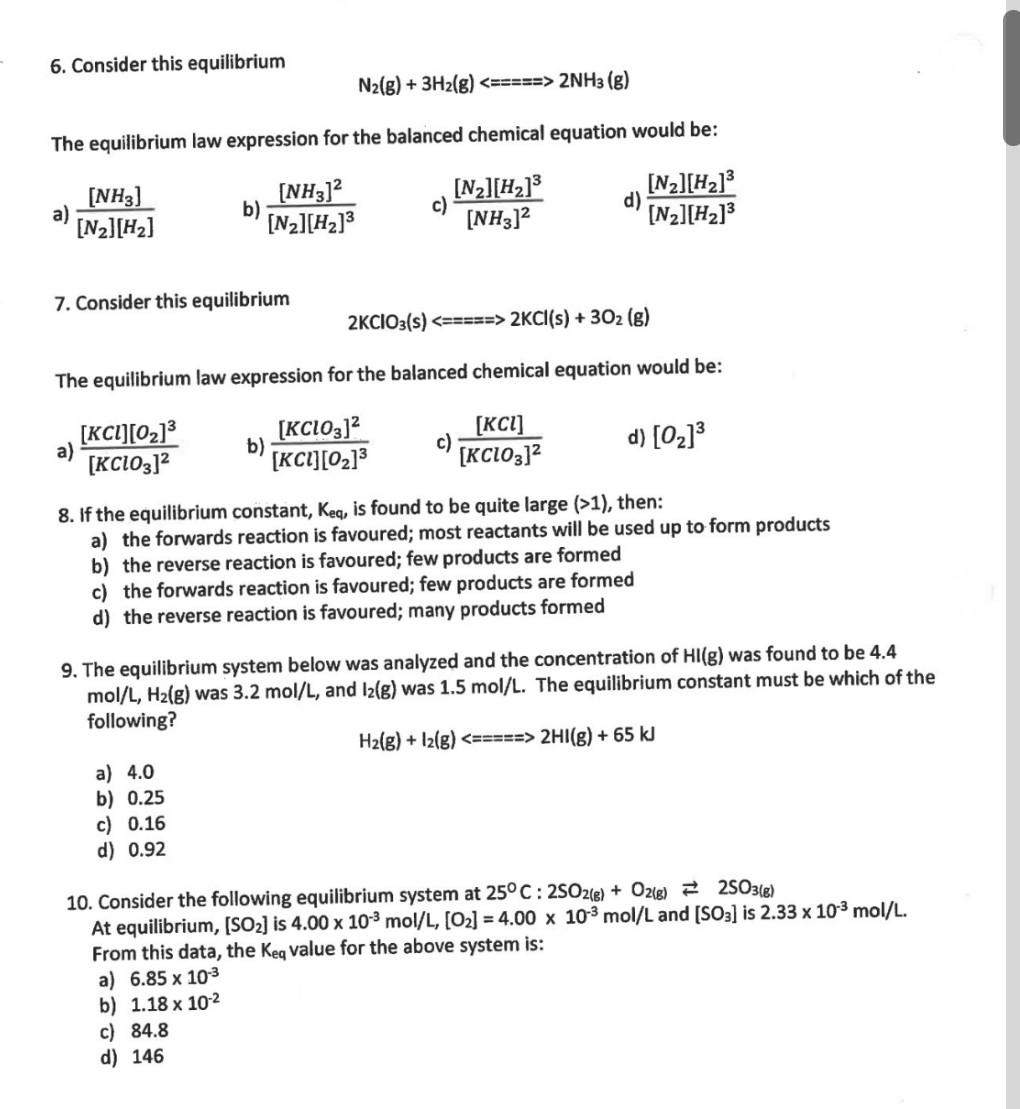


1. Consider the following equilibrium: H2O(g)+CO(g)H2(g)+CO2(g) A closed container is initially filled with H2O and CO. As the reaction proceeds towards quilibrium: a) [CO] and [CO2] both increase b) [CO] and [CO2] both decrease c) [CO] increases and [CO2] decreases d) [CO] decreases and [CO2] increases 2. Which property best represents a reaction that has reached equilibrium? a) The concentration of reactants is equal to the concentration of products. b) The rate of the forward reaction is equal to the rate of the reverse reaction. c) Both A and B. d) Neither A nor B. 3. A system at equilibrium is said to be dynamic because at equilibrium the: a) Temperature does not change b) Forward and reverse reactions continue to occur c) No observable or measurable changes occur d) Concentrations of reactants and products are constant 4. Which of the following systems could be at equilibrium? a) a pot of boiling water without a lid b) H2O(l)H2O(g) in a closed flask c) I2(s) is placed in an open jar d) All of the above could be at equilibrium. 5. Consider the following: 2NO2(g)N2O4(g) Brown Colourless NO2 is placed in a flask at a constant temperature. Which of the following is true as the system approaches equilibrium? a) The colour gets darker as [NO2] increases. b) The colour gets lighter as [NO2] decreases. c) The colour gets darker as [N2O4] increases. d) The colour gets lighter as [N2O4] decreases. 16. Which of the following reactions will shift left when pressure is increased and when temperature is decreased? a) N2(g)+2O2(g)+ heat 2NO2(g) b) N2(g)+3H2(g)2NH3(g)+ heat c) CH4(g)+H2O(g)+ heat CO(g)+3H2(g) d) CS2(g)+4H2(g)CH4(g)+2H2S(g)+ heat 17. For the equilibrium system below, which of the following would result if we were to add a catalyst? A+2BC+D a) an equilibrium shift to the right b) an equilibrium shift to the left c) no change in the position of equilibrium d) impossible to determine 18. Which of the following is true regarding the equilibrium constant if more CO is added to the following equilibrium system? a) Keq remains the same b) Keqmust increase c) Keq must decrease d) Keq is unchanged but the temperature increases 19. Consider the following equilibrium: C(s)+2H2(g)CH4(g)+74kJ When a small amount of solid C is added to the system: a) [H2] decreases b) [CH4] increases c) The temperature increases d) All concentrations remain constant 20. For the equilibrium system below, which of the following would result in an increase in the quantity of Cl2(g) ? a) adding some PCl3(g) b) removing some PCl5(g) c) decreasing temperature d) increasing the volume of the container 21. For the equilibrium system below, which of the following would result in a decrease in the quantity of PCl5(g) ? a) increasing temperature b) adding some Cl2(g) c) decreasing the volume of the container d) injecting some He gas 6. Consider this equilibrium N2(g)+3H2(g)====2NH3(g) The equilibrium law expression for the balanced chemical equation would be: a) [N2][H2][NH3] b) [N2][H2]3[NH3]2 c) [NH3]2[N2][H2]3 d) [N2][H2]3[N2][H2]3 7. Consider this equilibrium 2KClO3(s)1), then: a) the forwards reaction is favoured; most reactants will be used up to form products b) the reverse reaction is favoured; few products are formed c) the forwards reaction is favoured; few products are formed d) the reverse reaction is favoured; many products formed 9. The equilibrium system below was analyzed and the concentration of HI(g) was found to be 4.4 mol/L,H2(g) was 3.2mol/L, and I2(g) was 1.5mol/L. The equilibrium constant must be which of the following? H2(g)+I2(g)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


