Question
For the following process, determine the change in entropy for the system (AS sys), the surrounding (A.S sys), and the universe (AS univ). Then
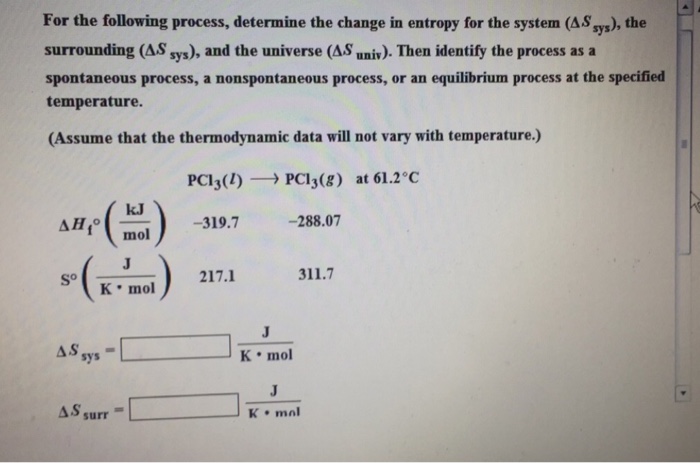
For the following process, determine the change in entropy for the system (AS sys), the surrounding (A.S sys), and the universe (AS univ). Then identify the process as a spontaneous process, a nonspontaneous process, or an equilibrium process at the specified temperature. (Assume that the thermodynamic data will not vary with temperature.) PC13(1) PC13(g) at 61.2C So J K mol AS sys kJ mol AS surr -319.7 217.1 -288.07 J K mol . 311.7 J K mal
Step by Step Solution
3.51 Rating (175 Votes )
There are 3 Steps involved in it
Step: 1
The image youve provided contains a question regarding thermodynamics Specifically its asking to calculate the change in entropy for a system Ssys sur...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: James S. Walker
5th edition
978-0133498493, 9780321909107, 133498492, 0321909100, 978-0321976444
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



