Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Draw a structural formula and give (when you can) an alternative name for: (a) ethylene bromide (c) bromoethylene (e) propylene glycol (f) propylene
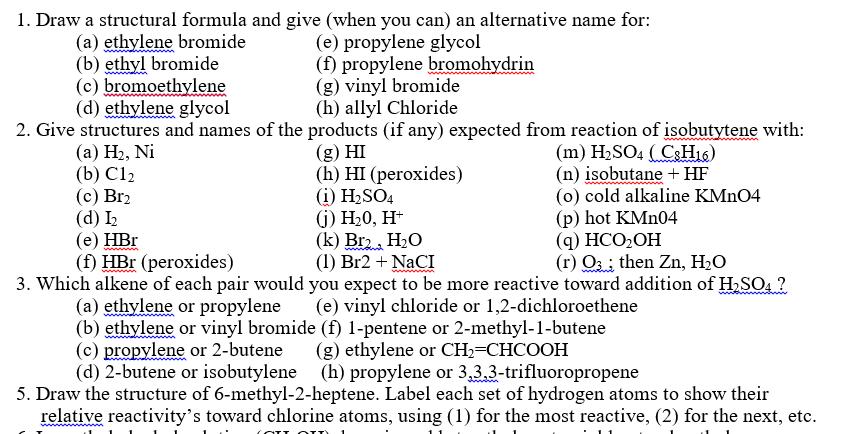
1. Draw a structural formula and give (when you can) an alternative name for: (a) ethylene bromide (c) bromoethylene (e) propylene glycol (f) propylene bromohydrin (g) vinyl bromide (h) allyl Chloride 2. Give structures and names of the products (if any) expected from reaction of isobutytene with: (b) ethyl bromide (d) ethylene glycol (a) H, Ni (b) C12 (c) Br2 (d) I (e) HBr (f) HBr (peroxides) (g) HI (h) HI (peroxides) (i) H2SO4 (j) H0, H+ (k) Br2 HO (1) Br2 + NaCI (m) H2SO4 (C8H16) (n) isobutane + HF (o) cold alkaline KMnO4 (p) hot KMn04 (q) HCOOH (r) Othen Zn, HO 3. Which alkene of each pair would you expect to be more reactive toward addition of H2SO4 ? (a) ethylene or propylene (e) vinyl chloride or 1,2-dichloroethene (b) ethylene or vinyl bromide (f) 1-pentene or 2-methyl-1-butene (c) propylene or 2-butene (d) 2-butene or isobutylene (g) ethylene or CH2=CHCOOH (h) propylene or 3,3,3-trifluoropropene 5. Draw the structure of 6-methyl-2-heptene. Label each set of hydrogen atoms to show their relative reactivity's toward chlorine atoms, using (1) for the most reactive, (2) for the next, etc.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


