Question
1. Draw all the forms of Tyrosine at pH 10.5. I have the predominant form and the protonation and deprotonation values written out, but I'm
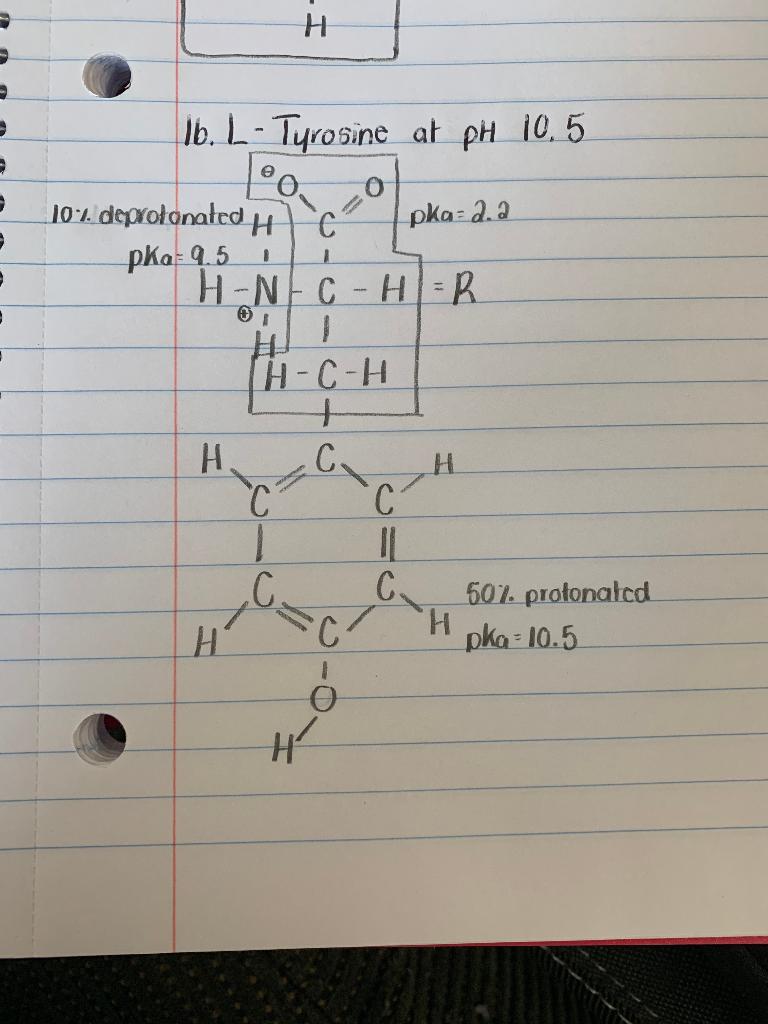
1. Draw all the forms of Tyrosine at pH 10.5. I have the predominant form and the protonation and deprotonation values written out, but I'm not sure how to draw the other forms now.
2. Draw the peptide chain arginyl-alanyl-histidyl-cysteinyl-isoleucyl-asparagine at pH 7
Ib. L-Tyrosine at pH 10,5 10:1. deprotonatedH pKa 9.5 H-N C-H= R pka= a.a H-C-H H. C. C. H. C. C. C. 507. protonated H. pka 10.5
Step by Step Solution
3.32 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Compensation
Authors: George Milkovich, Jerry Newman, Barry Gerhart
11th edition
9780077512903, 007802949X, 77512901, 978-0078029493
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



