Question
1. Draw the C4HBr isomer which gives rise to each spectrum below. (Note: each spectrum (a-c) shows a different isomer!) 8. 8 6 4
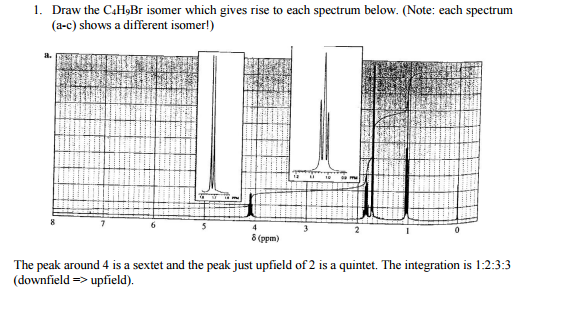
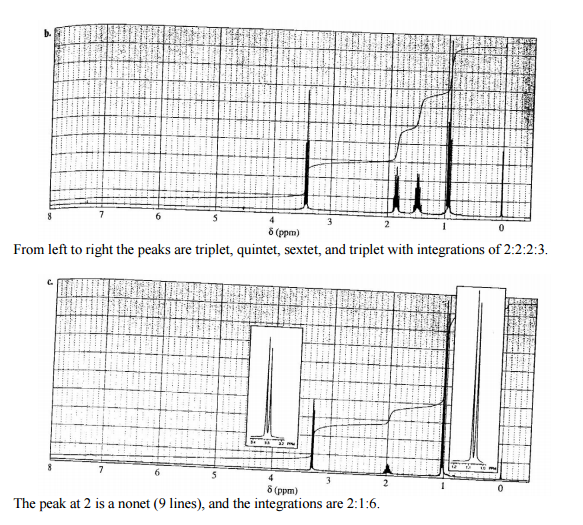
1. Draw the C4HBr isomer which gives rise to each spectrum below. (Note: each spectrum (a-c) shows a different isomer!) 8. 8 6 4 8 (ppm) 0 The peak around 4 is a sextet and the peak just upfield of 2 is a quintet. The integration is 1:2:3:3 (downfield => upfield). 7 4 0 8 (ppm) From left to right the peaks are triplet, quintet, sextet, and triplet with integrations of 2:2:2:3. 7 3 8 (ppm) The peak at 2 is a nonet (9 lines), and the integrations are 2:1:6. IN 2
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Paula Yurkanis Bruice
4th edition
131407481, 978-0131407480
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



