Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Equal concentrations of acrylonitrile (M.W. =55g/mol ) and methyl methacrylate (M.W.= 100g/mol) are each polymerized in separate reactors at 60C with equal concentrations of
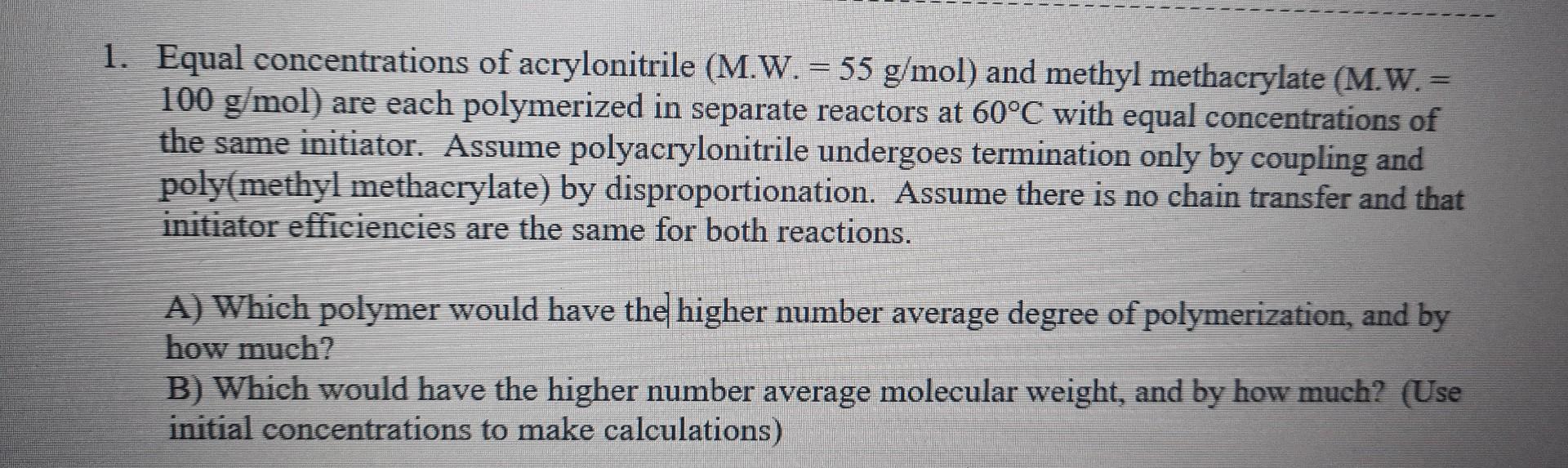
1. Equal concentrations of acrylonitrile (M.W. =55g/mol ) and methyl methacrylate (M.W.= 100g/mol) are each polymerized in separate reactors at 60C with equal concentrations of the same initiator. Assume polyacrylonitrile undergoes termination only by coupling and poly(methyl methacrylate) by disproportionation. Assume there is no chain transfer and that initiator efficiencies are the same for both reactions. A) Which polymer would have the higher number average degree of polymerization, and by how much? B) Which would have the higher number average molecular weight, and by how much? (Use initial concentrations to make calculations)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


