Question: 1 Eutectic phase diagram: For the Pb-Sn diagram shown below, Composition (at% Sn) 20 40 60 80 100 327.C 600 300 Liquid 500 2320 a+L
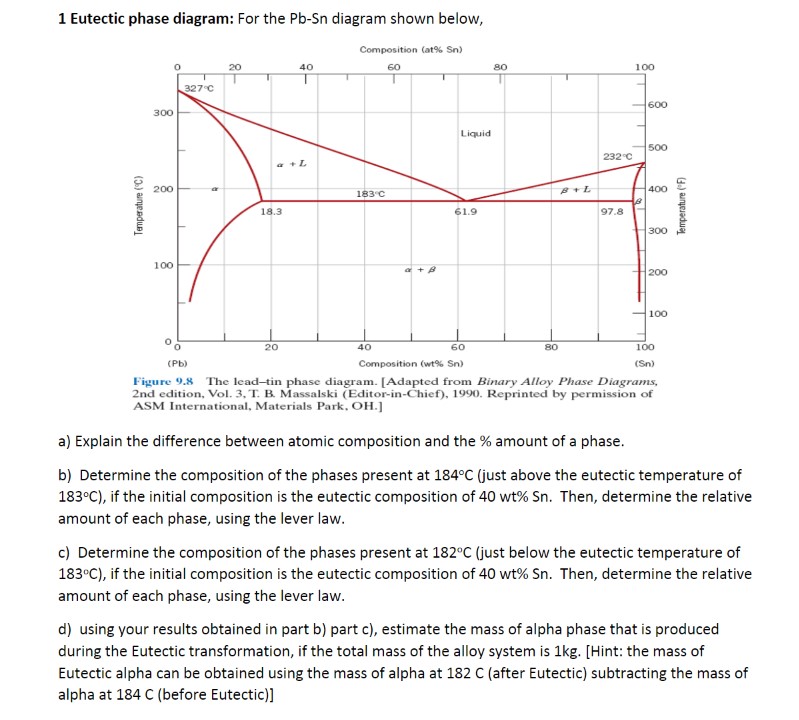
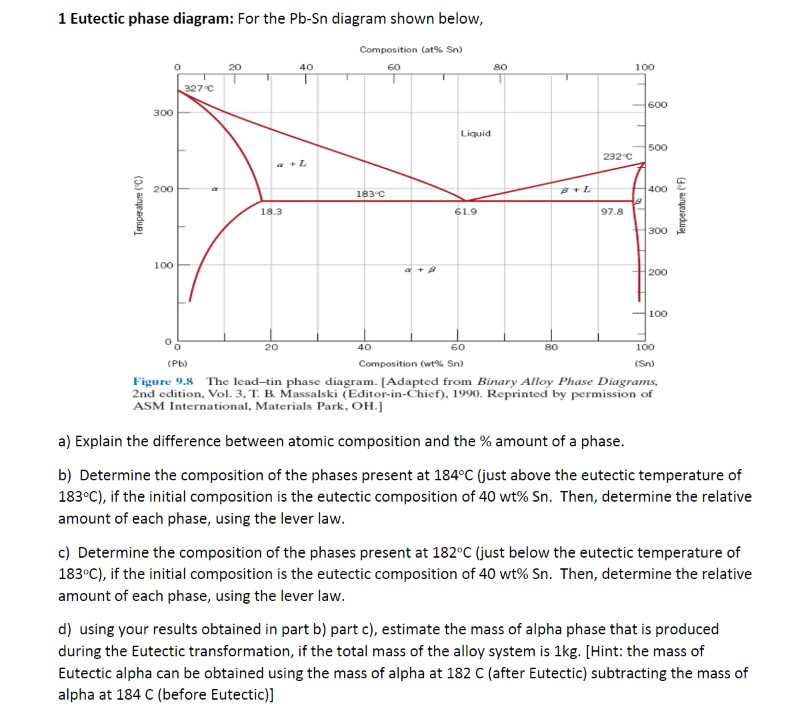
1 Eutectic phase diagram: For the Pb-Sn diagram shown below, Composition (at% Sn) 20 40 60 80 100 327.C 600 300 Liquid 500 2320 a+L 200 183 C 400 Temperature ( F Temperature (C) 18.3 61.9 97.8 300 100 200 -100 20 40 60 80 100 (Pb) Composition (wt% Sn) (Sn) Figure 9.8 The lead-tin phase diagram. [Adapted from Binary Alloy Phase Diagrams, 2nd edition, Vol. 3, T. B. Massalski (Editor-in-Chief), 1990. Reprinted by permission of ASM International, Materials Park. OH.] a) Explain the difference between atomic composition and the % amount of a phase. b) Determine the composition of the phases present at 184 C (just above the eutectic temperature of 183.C), if the initial composition is the eutectic composition of 40 wt% Sn. Then, determine the relative amount of each phase, using the lever law. c) Determine the composition of the phases present at 182C (just below the eutectic temperature of 183"C), if the initial composition is the eutectic composition of 40 wt% Sn. Then, determine the relative amount of each phase, using the lever law. d) using your results obtained in part b) part c), estimate the mass of alpha phase that is produced during the Eutectic transformation, if the total mass of the alloy system is 1kg. [Hint: the mass of Eutectic alpha can be obtained using the mass of alpha at 182 C (after Eutectic) subtracting the mass of alpha at 184 C (before Eutectic)]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


