Question
For a self-complementary DNA oligonucleotide, the melting temperature is monitored as a function of total strand concentration: T(K) Strand 315 318 321 5.00 X
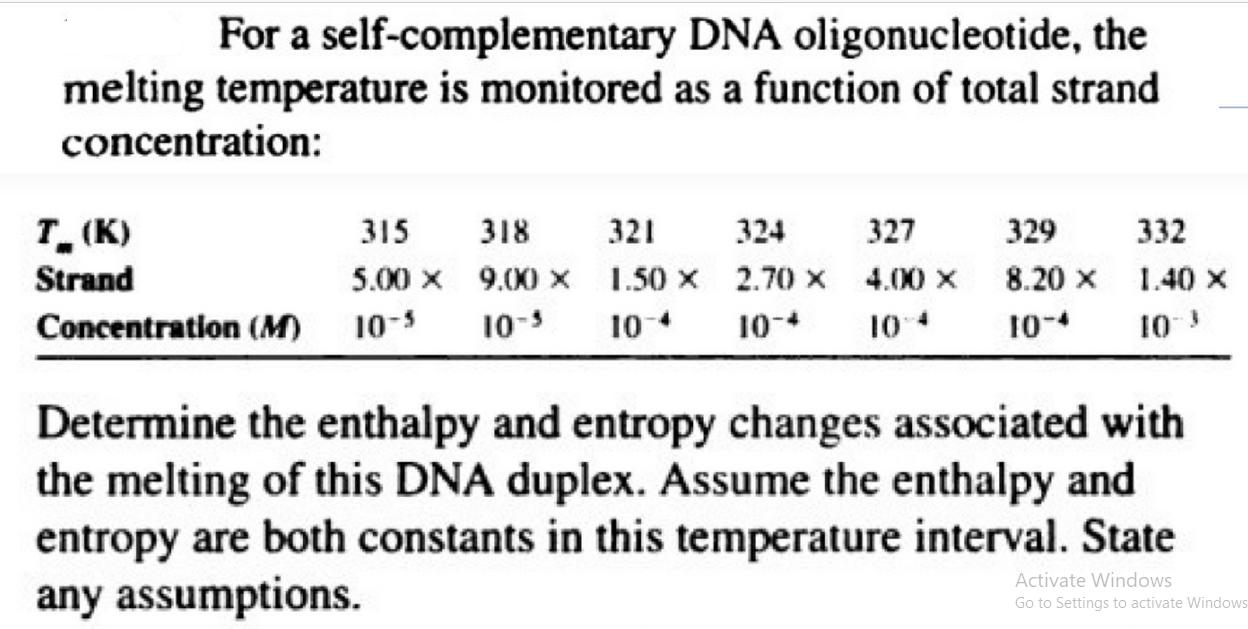
For a self-complementary DNA oligonucleotide, the melting temperature is monitored as a function of total strand concentration: T(K) Strand 315 318 321 5.00 X 9.00 X 1.50 X Concentration (M) 10-5 10-5 10-4 324 2.70 X 10-* 327 4.00 X 10:4 329 8.20 X 10-4 332 1.40 X 10-3 Determine the enthalpy and entropy changes associated with the melting of this DNA duplex. Assume the enthalpy and entropy are both constants in this temperature interval. State any assumptions. Activate Windows Go to Settings to activate Windows
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION To determine the enthalpy and entropy changes associated with the melting of the DNA duplex we need to use the following equation H U PV where H is the enthalpy change U is the internal energ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Modern Control Systems
Authors: Richard C. Dorf, Robert H. Bishop
12th edition
136024580, 978-0136024583
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



