Question
1. First evaluate the reactants side: Start with CH H 2 +1 +2 CH number of atoms oxidation state total charge C 2 -1
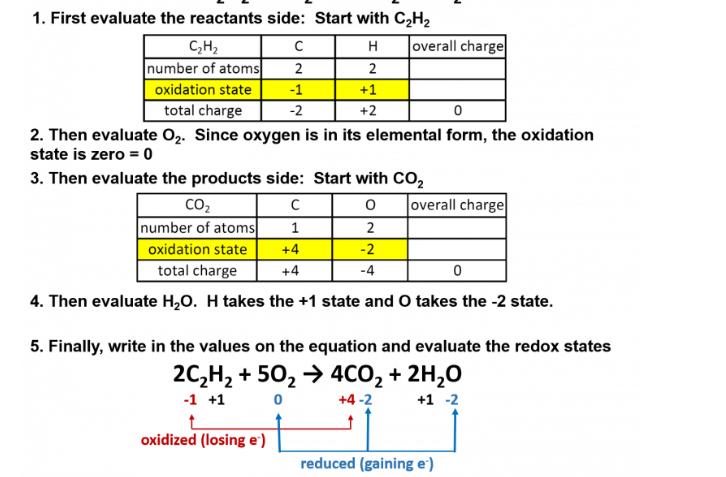
1. First evaluate the reactants side: Start with CH H 2 +1 +2 CH number of atoms oxidation state total charge C 2 -1 -2 overall charge 0 2. Then evaluate O. Since oxygen is in its elemental form, the oxidation state is zero = 0 3. Then evaluate the products side: Start with CO 0 1 2 +4 -2 +4 -4 CO number of atoms oxidation state total charge 0 4. Then evaluate HO. H takes the +1 state and O takes the -2 state. oxidized (losing e') overall charge 5. Finally, write in the values on the equation and evaluate the redox states 2CH +50 4CO + 2HO -1 +1 0 +4-2 +1 -2 reduced (gaining e')
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer The given chemical equation is 2CH 5O 4CO 2HO To determine which substance is oxidized and wh...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



