Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. For the reaction: A+ 3B 2C+ D, what is the order of the above reaction with respect to [B]? Initial Rate (M-s) [A]
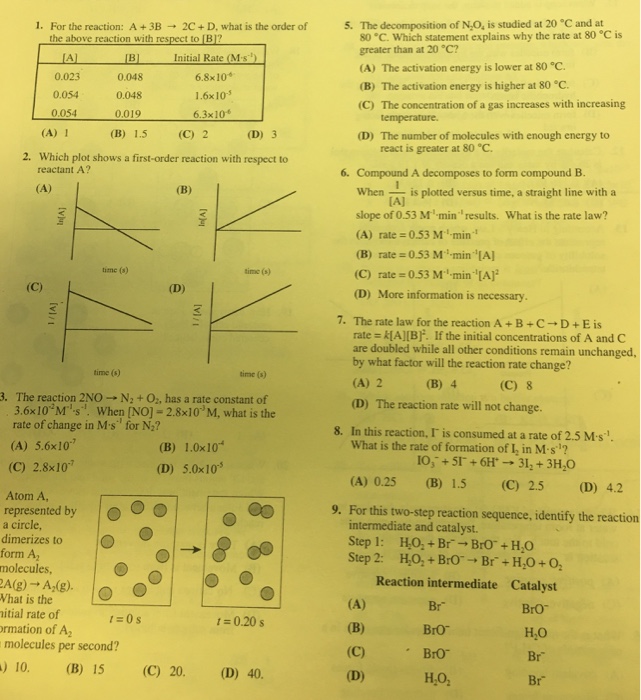
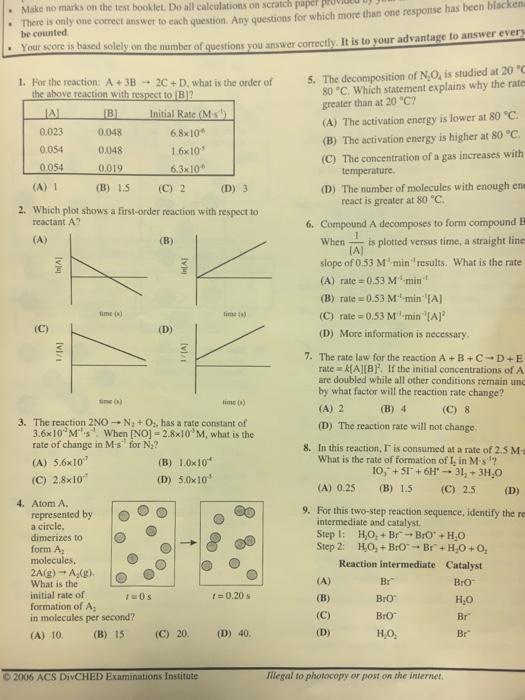
1. For the reaction: A+ 3B 2C+ D, what is the order of the above reaction with respect to [B]? Initial Rate (M-s) [A] 0.023 0.054 0.054 [B] 0.048 0.048 0.019 (B) 1.5 (D) 3 2. Which plot shows a first-order reaction with respect to reactant A? (A) (A) 1 O 3 [v] (vi/1 Atom A, represented by a circle, dimerizes to form A molecules, time (s) 2A(g) A(g). What is the nitial rate of ormation of A molecules per second? ) 10. (B) 15 1=0s (C) 2 (B) (D) 6.8x10 1.6x10-5 6.3x10 time (s) time (s) 3. The reaction 2NO-N + O, has a rate constant of 3.6x10 M-s. When [NO] - 2.8x10 M, what is the rate of change in M's for N? (A) 5.6x107 (C) 2.8x107 In(A) (C) 20. [vi/1 (B) 1.0x10 (D) 5.0x105 time (s) 1 = 0.20 s (D) 40. 5. The decomposition of N.O, is studied at 20 C and at 80 C. Which statement explains why the rate at 80 C is greater than at 20 C? (A) The activation energy is lower at 80 C. (B) The activation energy is higher at 80 C. (C) The concentration of a gas increases with increasing temperature. (D) The number of molecules with enough energy to react is greater at 80 C. 6. Compound A decomposes to form compound B. When is plotted versus time, a straight line with a [A] slope of 0.53 M-min results. What is the rate law? (A) rate=0.53 M min (B) rate=0.53 M min [A] (C) rate=0.53 M min [A] (D) More information is necessary. 7. The rate law for the reaction A+B+C+D+E is rate = k[A][B]. If the initial concentrations of A and C are doubled while all other conditions remain unchanged, by what factor will the reaction rate change? (A) 2 (B) 4 (C) 8 (D) The reaction rate will not change. 8. In this reaction, I is consumed at a rate of 2.5 M-s''. What is the rate of formation of I, in M-s? 10, +51 +6H 31 + 3HO (B) 1.5 (C) 2.5 (A) 0.25 (D) 4.2 9. For this two-step reaction sequence, identify the reaction intermediate and catalyst. Step 1: Step 2: HO+ BrBrO + HO HO+ BrO Br + HO +0 Reaction intermediate Catalyst BrO HO Br Br (A) (B) (C) (D) Br Bro BrO HO . Make no marks on the test booklet. Do all calculations on scratch paper pro There is only one correct answer to each question. Any questions for which more than one response has been blackens be counted Your score is based solely on the number of questions you answer correctly. It is to your advantage to answer every 1. For the reaction: A+3B2C+ D, what is the order of the above reaction with respect to [B]? Initial Rate (M-s') JA 0.023 0.054 0.054 [B] 0.048 0.048 0.019 (B) 1.5 (D) 3 2. Which plot shows a first-order reaction with respect to reactant A? (A) (A) 1 O (vu [VI/1) 4. Atom A. represented by a circle, dimerizes to form A molecules, time() 2A(g) A(g). What is the (1) initial rate of formation of A in molecules per second? (A) 10. (B) 15 6.8x10 1.6x10 6.3x10 time() 3. The reaction 2NO-N + O, has a rate constant of 3.6x10 Ms. When [NO] -2.8x10 M, what is the rate of change in M-s for N? (A) 5.6x10 (C) 2.8x10 r=0 s (C) 2 (B) (D) In[A] M (B) 1.0x10 (D) 5.0x10 (C) 20. time) 2006 ACS DivCHED Examinations Institute 000 O 1=0.20 s (D) 40. 5. The decomposition of N,O, is studied at 20 "C 80 C. Which statement explains why the rates greater than at 20 "C? (A) The activation energy is lower at 80 C. (B) The activation energy is higher at 80 C. (C) The concentration of a gas increases with temperature. (D) The number of molecules with enough ene react is greater at 80 C. When 6. Compound A decomposes to form compound E is plotted versus time, a straight lines [A] slope of 0.53 M-min" results. What is the rate (A) rate=0.53 M-min (B) rate=0.53 M min [A] (C) rate=0.53 M-min "[A] (D) More information is necessary. 7. The rate law for the reaction A+B+C-D+E rate k[A][B]. If the initial concentrations of A are doubled while all other conditions remain unc by what factor will the reaction rate change? (A) 2 (B) 4 (C) 8 (D) The reaction rate will not change. 8. In this reaction, I is consumed at a rate of 2.5 Ma What is the rate of formation of I, in M-s? 10, +51 +6H 31, + 3HO (C) 2.5 (A) 0.25 (B) 1.5 (D) 9. For this two-step reaction sequence, identify the re intermediate and catalyst. Step 1: Step 2: (A) (B) (C) (D) H,O,+Br-BrO+HO H,O,+BrO Br + HO +0 Reaction intermediate Catalyst BrO HO Br Br Bro Bro HO, Illegal to photocopy or post on the internet. Br
Step by Step Solution
★★★★★
3.46 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
Here are the answers to the chemistry questions 1 The order of the reaction with respect to B is 1 T...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


