Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Gas phase decomposition of di-t-butyl peroxide is monitored in a batch reactor of constant volume at isothermal conditions of 170C. (CH3)3COOC(CH3)3C2H6+2CH3COCH3 The run is
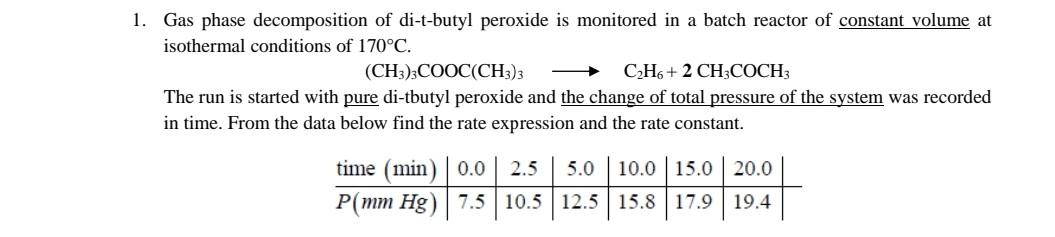
1. Gas phase decomposition of di-t-butyl peroxide is monitored in a batch reactor of constant volume at isothermal conditions of 170C. (CH3)3COOC(CH3)3C2H6+2CH3COCH3 The run is started with pure di-tbutyl peroxide and the change of total pressure of the system was recorded in time. From the data below find the rate expression and the rate constant
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


