Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Identify the element whose last electron would have the following four quantum numbers: a. 3,1,1,+1/2 b. 4,2,+1,+1/2 c. 6,1,0,1/2 2. Label each of the
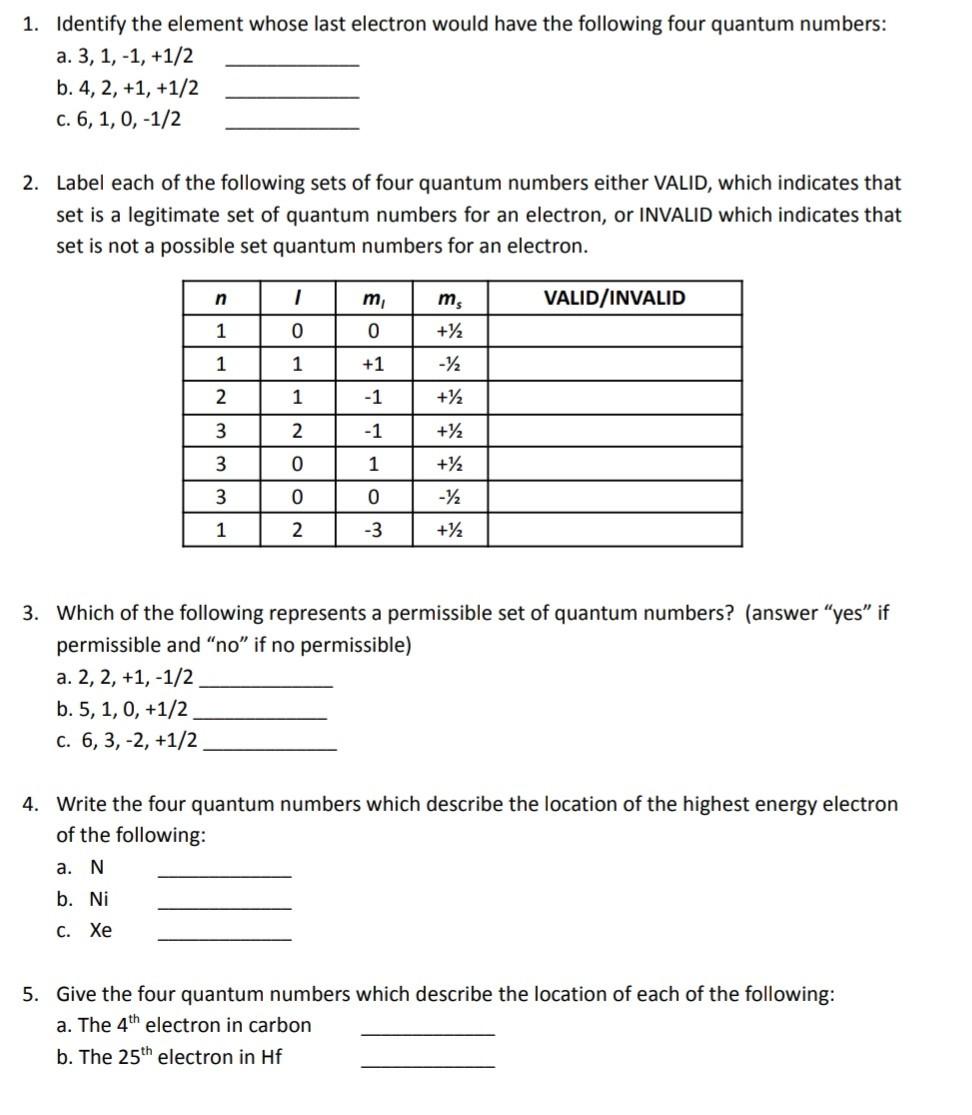
1. Identify the element whose last electron would have the following four quantum numbers: a. 3,1,1,+1/2 b. 4,2,+1,+1/2 c. 6,1,0,1/2 2. Label each of the following sets of four quantum numbers either VALID, which indicates that set is a legitimate set of quantum numbers for an electron, or INVALID which indicates that set is not a possible set quantum numbers for an electron. 3. Which of the following represents a permissible set of quantum numbers? (answer "yes" if permissible and "no" if no permissible) a. 2,2,+1,1/2 b. 5,1,0,+1/2 c. 6,3,2,+1/2 4. Write the four quantum numbers which describe the location of the highest energy electron of the following: a. N b. Ni C. Xe 5. Give the four quantum numbers which describe the location of each of the following: a. The 4th electron in carbon b. The 25th electron in Hf
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


