Question
1. Identify the oxidizing agent and the reducing agent in the reaction. 8H*(aq) + 6CI (aq) + Sn(s) + 4NO3 (aq) SnCl (aq) +
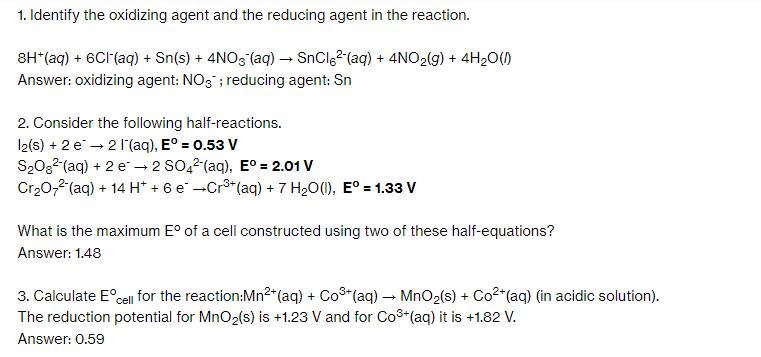
1. Identify the oxidizing agent and the reducing agent in the reaction. 8H*(aq) + 6CI (aq) + Sn(s) + 4NO3 (aq) SnCl (aq) + 4NO(g) + 4H0(1) Answer: oxidizing agent: NO3; reducing agent: Sn 2. Consider the following half-reactions. 12(s) + 2 e 2 l'(aq), E = 0.53 V SO (aq) + 2 e 2 SO42 (aq), E = 2.01 V CrO7 (aq) + 14 H+ + 6 e Cr+ (aq) + 7 HO(1), E = 1.33 V What is the maximum E of a cell constructed using two of these half-equations? Answer: 1.48 3. Calculate Ecell for the reaction:Mn+ (aq) + Co+ (aq) MnO(s) + Co+ (aq) (in acidic solution). The reduction potential for MnO(s) is +1.23 V and for Co+ (aq) it is +1.82 V. Answer: 0.59
Step by Step Solution
There are 3 Steps involved in it
Step: 1
In the given reaction 8Haq 6Claq Sns 4NO3aq SnCl2aq 4NO...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



