Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) If a black solid is produced by the addition of copper(II) nitrate to the supernatant created in part 1, this indicates which ion is


1)
If a black solid is produced by the addition of copper(II) nitrate to the supernatant created in part 1, this indicates which ion is present in the solution?
iodide
phosphate
carbonate
chloride
nitrate
sulfide
2.)
The supernatant created in part 4 may contain which ions? (select all that apply)
phosphate
chloride
carbonate
iodide
nitrate
sulfide
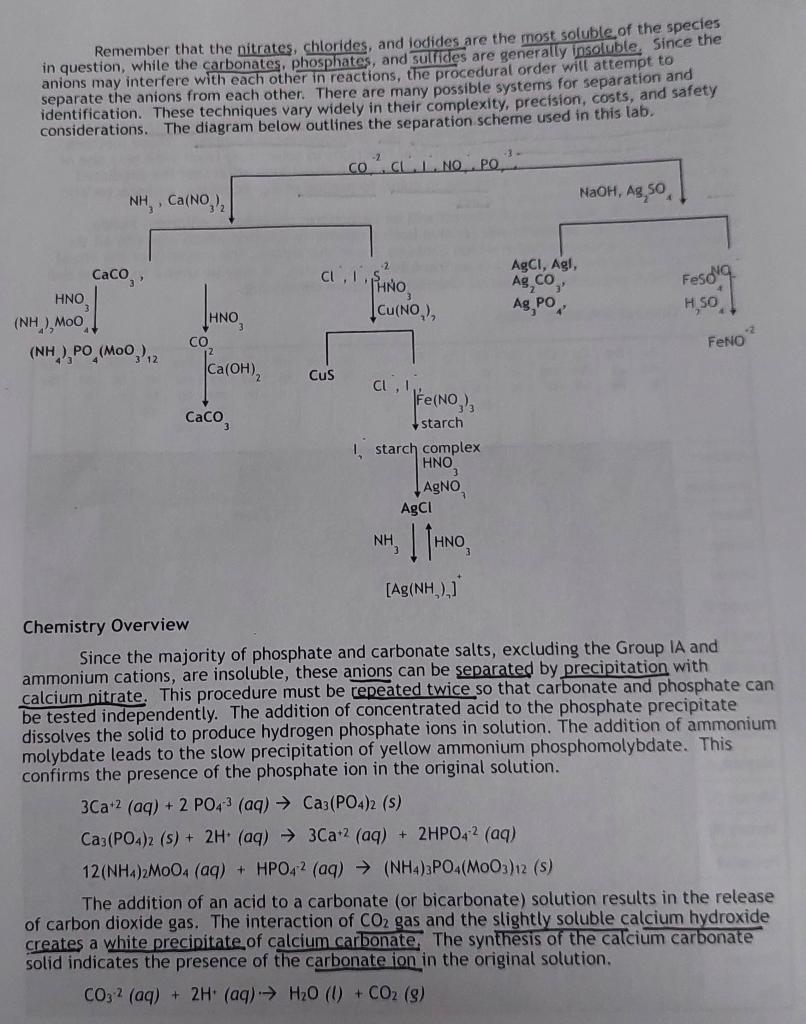
Remember that the nitrates, chlorides, and lodides are the most soluble of the species in question, while the carbonates, phosphates, and sulfides are generally insoluble. since the anions may interfere with each other in reactions, the procedural order will attempt to separate the anions from each other. There are many possible systems for separation and identification. These techniques vary widely in their complexity, precision, costs, and safety considerations. The diagram below outlines the separation scheme used in this lab. Chemistry Overview Since the majority of phosphate and carbonate salts, excluding the Group IA and ammonium cations, are insoluble, these anions can be separated by precipitation with calcium nitrate. This procedure must be repeated twice so that carbonate and phosphate can be tested independently. The addition of concentrated acid to the phosphate precipitate dissolves the solid to produce hydrogen phosphate ions in solution. The addition of ammonium molybdate leads to the slow precipitation of yellow ammonium phosphomolybdate. This confirms the presence of the phosphate ion in the original solution. 3Ca+2(aq)+2PO43(aq)Ca3(PO4)2(s)Ca3(PO4)2(s)+2H+(aq)3Ca+2(aq)+2HPO42(aq)12(NH4)2MOO4(aq)+HPO42(aq)(NH4)3PO4(MOO3)12(s) The addition of an acid to a carbonate (or bicarbonate) solution results in the release of carbon dioxide gas. The interaction of CO2 gas and the slightly soluble calcium hydroxide creates a white precipitate of calcium carbonate the synthesis of the calcium carbonate solid indicates the presence of the carbonate ion in the original solution. CO32(aq)+2H+(aq)H2O(l)+CO2(g) CO2(g)+Ca(OH)2(aq)CaCO3(s)+H2O(l) Since most sulfide salts are insoluble, copper (II) nitrate can be used to precipitate the sulfide ion as a readily identifiable black precipitate in an acidic solution. Cu+2(aq)+S2(aq)CuS(s) The simplest precipitates of the halide ions are the silver salts. Since chloride and iodide may be present, the iodide is oxidized to the triodide ion prior to the addition of silver. The triiodide ion forms a deep blue complex with starch, a carbohydrate polymer [(C6H10O5)n]. Now that the iodide ion has been isolated, the chloride ion can be detected aa the white silver chloride precipitate. The solid salt can be eacted with ammonia to form the diamminesilver (I) complex ion. Further addition of acid returns the solid salt. 2Fe+3(aq)+3I(aq)2Fe+2(aq)+I3(aq)I3(aq)+starch(aq)I3-starchcomplex(aq)Cl(aq)+Ag(aq)AgCl(s)AgCl(s)+2NH3(aq)[Ag(NH3)2](aq)+Cl(aq)[Ag(NH3)2]+(aq)+Cl(aq)+H+(aq)AgCl(s)+2NH4+(aq) Since all nitrate salts are soluble, the most common technique for identifying the nitrate ion in solution is the reduction to nitric oxide by iron (II) ions in concentrated sulfuric acid. The radical NO molecule combines with the iron (II) ions to form the FeNO+2 ion at the boundary between the more dense concentrated acid and aqueous layers. The ion is more stable at low temperatures, so an ice bath is used. The addition of sodium hydroxide and silver sulfate assist in removing the competing anions as a precipitate prior to the redox reaction. 3Fe+3(aq)+NO3(aq)+4H(aq)3Fe+2(aq)+NO(aq)+2H2O(l)Fe+2(aq)+NO(aq)FeNO2(aq) Procedure Students will obtain approximately 5mL of both the known reference solution and an assigned unknown. They should complete the following procedures on both samples simultaneously so they can compare known versus unknown results. Part 1: Separation of carbonate and phosphate ions Place approximately 1.5mL of the unknown solution in a small test tube. Using pH paper, test to see if the solution is acidic or basic. If acidic, add drops of 3MNH3 until the solution turns basic, then add 4 more drops of NH3. Add 1012 drops of 0.1MCa2(NO3)2 until precipitation is complete. Centrifuge the solution and decant the supernatant into a separate test tube. Label the precipitate as 1A and the supernatant as 1B. Rinse the precipitate twice with approximately 1mL of deionized water and discard the washings into a waste container. The solid contains any phosphate or carbonate ions while the supernatant contains the rest. Part 2: Test for the phosphate ion Use drops of 6MHNO3 to dissolve the precipitate, 1A. Add approximately 1mL of 0.5 M(NH4)2MoO4. Shake the solution gently and warm the test tube in the previously prepared warm water bath for one minute. Set aside and let stand for 15 minutes. The slow formation of a yellow precipitate indicates the presence of the phosphate ion. Part 3: Test for the carbonate ion Repeat Part 1. Label the precipitate 1C. The supernatant is the same as 1B so it may be saved for future testing or discarded. Dip a clean, dry glass rod into a saturated Ca(OH) solution. Add 3-5 drops of 6MHNO3 to the precipitate and into the test tube. Be very careful not to let the rod touch the walls of the CO2gasI solution. If the carbonate ion was present, the addition of nittic acid will rele of a milky solution on the which will rise to react with the Part 4: Test for the sulfide ion To the supernatant from Part I, 1B, add 24 drops of 6MHNO3 until pH paper indicates the solution is acidic. Next, add drops of 1MCCu(NO3)2 until precipitation is complete. Wait 2 minutes to see if a black precipitate forms. If so, this confirms the presence of the sulfide ion in solution. Centrifuge and label the precipitate 4A and the supernatant 4B. Part 5: Test for the iodide ion Transfer approximately 1mL of solution 4B into a new test tube and add approximately 5 drops of 0.2MFe2NO3)3. Shake gently and let stand for 3 minutes. The Fets the iodide ion. Part 6: Test for the chloride ion Using the same solution from Part 5, add 3 drops of 6MHNO3. Slowly add drops of 0.01MAgNO3 and look for a white precipitate. Centrifuge and discard the supernatant. For confirmation, the addition of 6MNH3 will dissolve the white precipitate and the addition of 6MHNO3 will reform the silver chloride solid. Part 7: Separation of nitrate from other anions Place approximately 1.5mL of the unknown solution into a small test tube, Add drops of 3MNaOH until pH paper indicates the solution is basic. Add drops of a saturated Ag2SO4 solution until the precipitation is complete. Centrifuge and discard the solid. Test for complete precipitation by adding a few more drops of silver sulfate. Centrifuge again if necessary. Part 8: Test for the nitrate ion Using a disposable pipet, transfer about 10 drops of the supernatant into a new test tube and acidify with 3MH2SO4. Add about 10 drops of a saturated FeSO solution and shake gently. Place the test tube in an ice bath at about a 45 angle. about 10 drops of concentrated H2SO4 along the inside of the test tube. Do interface between the solution! Watch carefully for the formation of a thin brown ring at the interface between the two solutions of different densities. The brown ring indicates the presence ofStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


