Question
1. In terms of structure and bonding, explain why graphite is used as a lubricant in machines. (3 marks) 2. Element U has atomic number
1. In terms of structure and bonding, explain why graphite is used as a lubricant in machines. (3 marks)
2. Element U has atomic number 12 while element V has atomic number 16. How do the melting points of their oxides compare? Explain. (3 marks)
3. Table 1 shows the properties of two chlorides, D and E.
Chloride | Melting Points(°C) | Electrical Conductivity(liquid) |
D | 1074 | Good |
E | 203 | Poor |
(a) State the type of bond present in:
(i) D.......................(1 mark)
(ii) E.......................(1 mark)
(b) Explain in terms of structure and bonding, the difference in electrical activity of the chlorides D and E. (1 mark)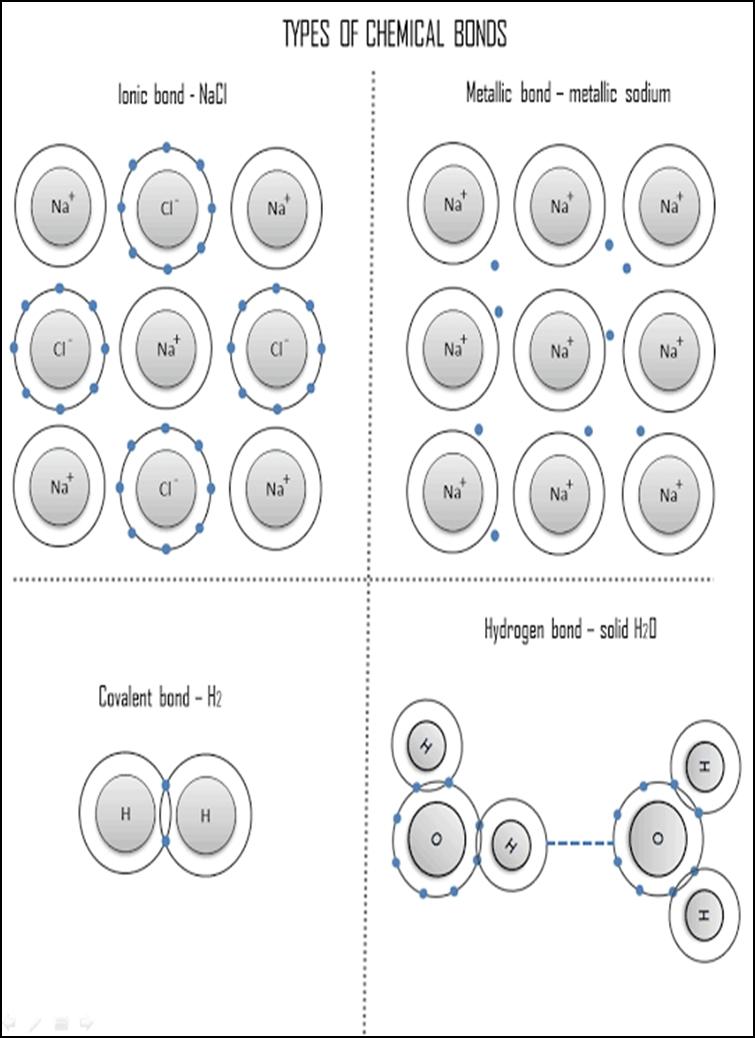
lonic band -NaCl O TYPES OF CHEMICAL BONDS Metallic bond metallic sodium Na Na Covalent bond - H2 Hydrogen bond solid HO
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


