Answered step by step
Verified Expert Solution
Question
1 Approved Answer
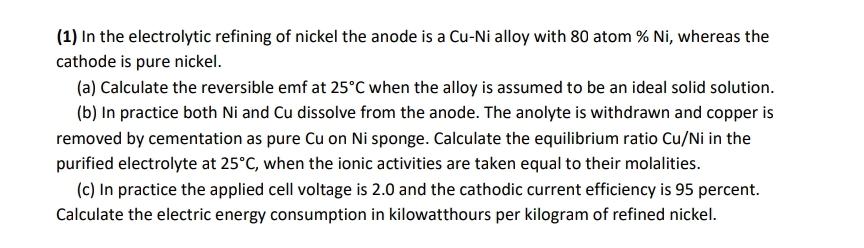
( 1 ) In the electrolytic refining of nickel the anode is a Cu - Ni alloy with 8 0 atom % N i ,
In the electrolytic refining of nickel the anode is a CuNi alloy with atom whereas the cathode is pure nickel.
a Calculate the reversible emf at when the alloy is assumed to be an ideal solid solution.
b In practice both and dissolve from the anode. The anolyte is withdrawn and copper is removed by cementation as pure on sponge. Calculate the equilibrium ratio in the purified electrolyte at when the ionic activities are taken equal to their molalities.
c In practice the applied cell voltage is and the cathodic current efficiency is percent. Calculate the electric energy consumption in kilowatthours per kilogram of refined nickel.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


