Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. It is easy to show that in fcc lattice four basal atoms: (000),(0, , ), (,0, ), (, ,0) form regular tetrahedron with
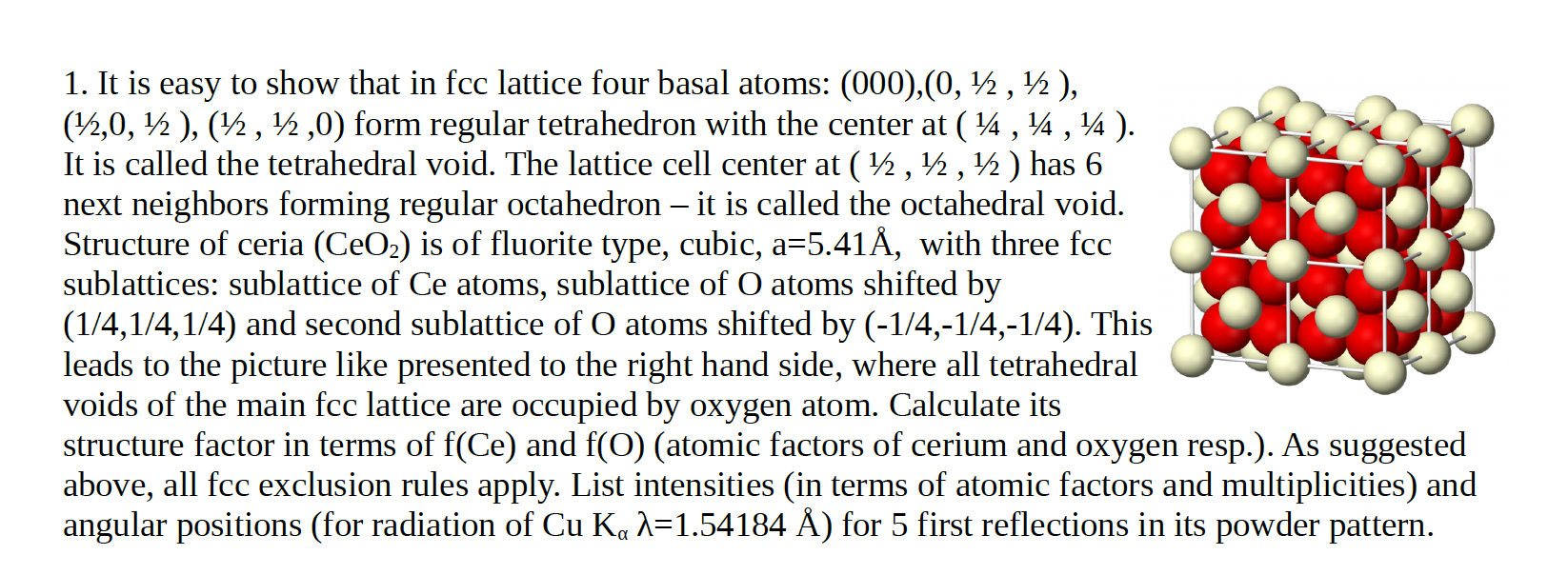
1. It is easy to show that in fcc lattice four basal atoms: (000),(0, , ), (,0, ), (, ,0) form regular tetrahedron with the center at ( , ). It is called the tetrahedral void. The lattice cell center at ( , , ) has 6 next neighbors forming regular octahedron it is called the octahedral void. Structure of ceria (CeO) is of fluorite type, cubic, a=5.41, with three fcc sublattices: sublattice of Ce atoms, sublattice of O atoms shifted by (1/4,1/4,1/4) and second sublattice of O atoms shifted by (-1/4,-1/4,-1/4). This leads to the picture like presented to the right hand side, where all tetrahedral voids of the main fcc lattice are occupied by oxygen atom. Calculate its structure factor in terms of f(Ce) and f(O) (atomic factors of cerium and oxygen resp.). As suggested above, all fcc exclusion rules apply. List intensities (in terms of atomic factors and multiplicities) and angular positions (for radiation of Cu Ka =1.54184 ) for 5 first reflections in its powder pattern.
Step by Step Solution
★★★★★
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Given Lattice parameter a 541 Wavelength of CuK radiation 154184 Step 1 Calculate the structure fact...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


