Question
1 kg of nitrogen (N2) gas at 140 K and 10 MPa undergoes a Joule-Thomson expansion to 1 MPa. For this purpose a throttle valve
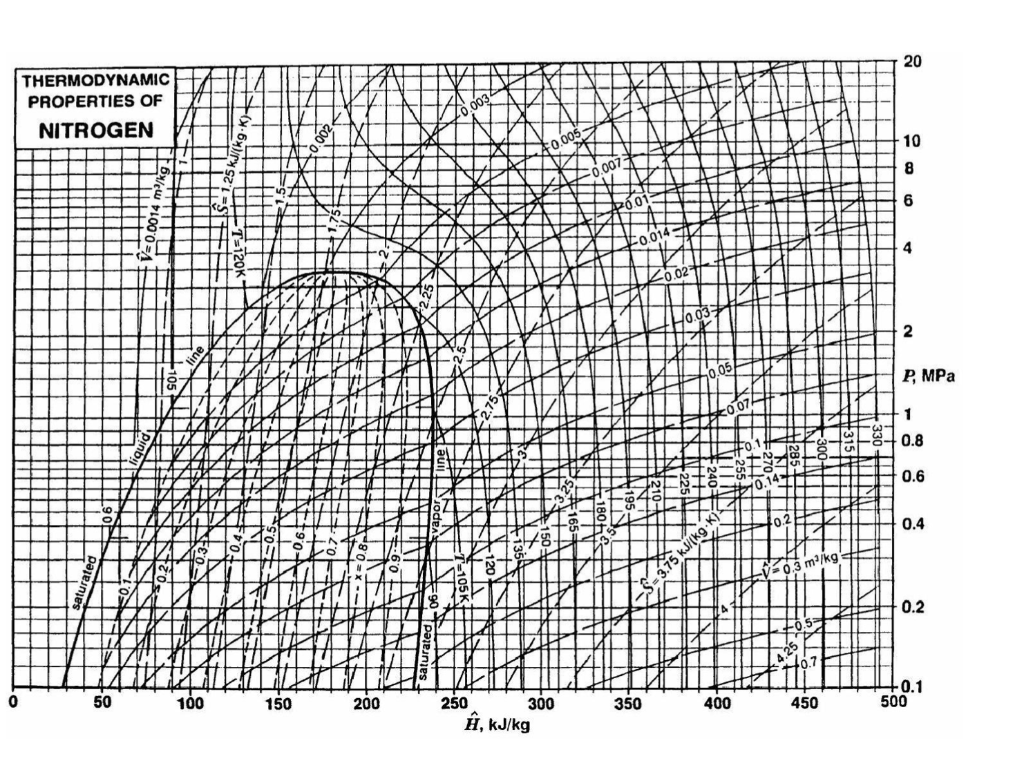
1 kg of nitrogen (N2) gas at 140 K and 10 MPa undergoes a Joule-Thomson expansion to 1 MPa. For this purpose a throttle valve is used and the expansion happens rapidly and adiabatically. For the above system,
a) Show that Joule-Thomson expansion is an isenthalpic process.
b) Calculate the enthalpy and temperature of nitrogen, and the fraction of vapour and liquid, leaving the throttle valve by using the nitrogen pressure-
enthalpy diagram provided.
c) Calculate how much heat needs to be removed from nitrogen after it has undergone Joule-Thomson expansion as explained in a) to achieve full
liquefaction at the same pressure, i.e. 1MPa.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


