Question
1 litre water 1kg i e 1000 g water d 1000 kg m 1000 18 55 55 moles of water So molarity of water 55
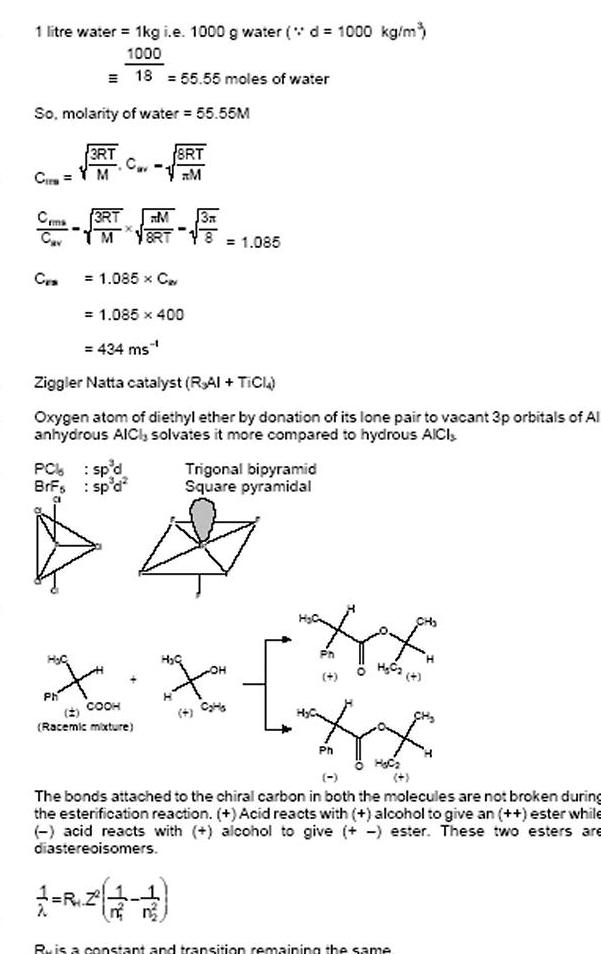
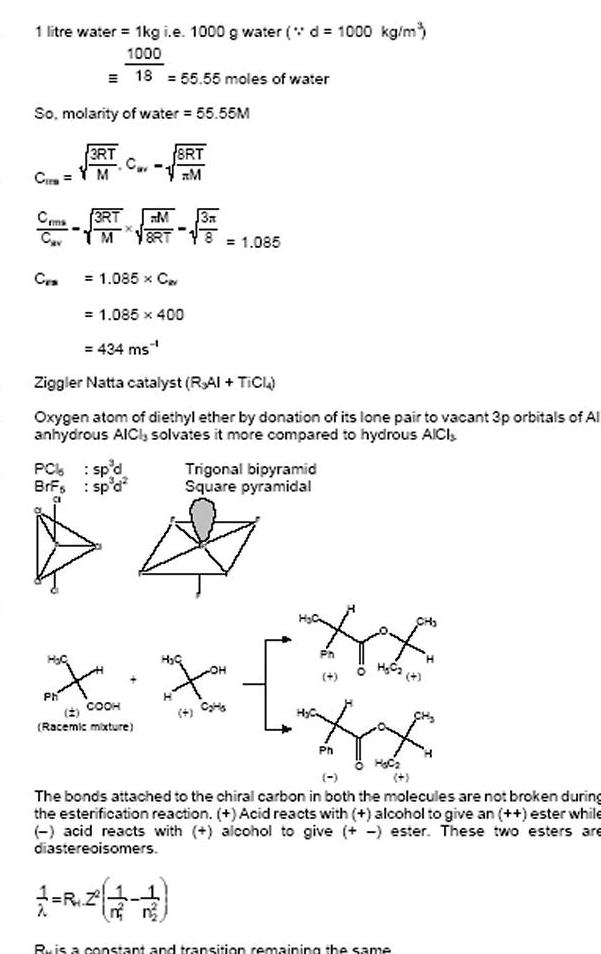
1 litre water 1kg i e 1000 g water d 1000 kg m 1000 18 55 55 moles of water So molarity of water 55 55M Cir Ceme Cav CES PC BrFs H C 3RT M Ph 3RT M M 8RT 1 085 x C 1 085 400 434 ms Ziggler Natta catalyst RAI TICI Oxygen atom of diethyl ether by donation of its lone pair to vacant 3p orbitals of Al anhydrous AICI solvates it more compared to hydrous AICI sp d sp d 8RT M 3A Racemic mixture 1 085 X X COOH Trigonal bipyramid Square pyramidal HE Ph H C2 4 Ph H C The bonds attached to the chiral carbon in both the molecules are not broken during the esterification reaction Acid reacts with alcohol to give an ester while acid reacts with alcohol to give ester These two esters are diastereoisomers Ru is a constant and transition remaining the same
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


