Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Osmotic Swelling of Lipid Vesicles A lipid bilayer is a thin polar membrane made of two layers of lipid molecules, which form a
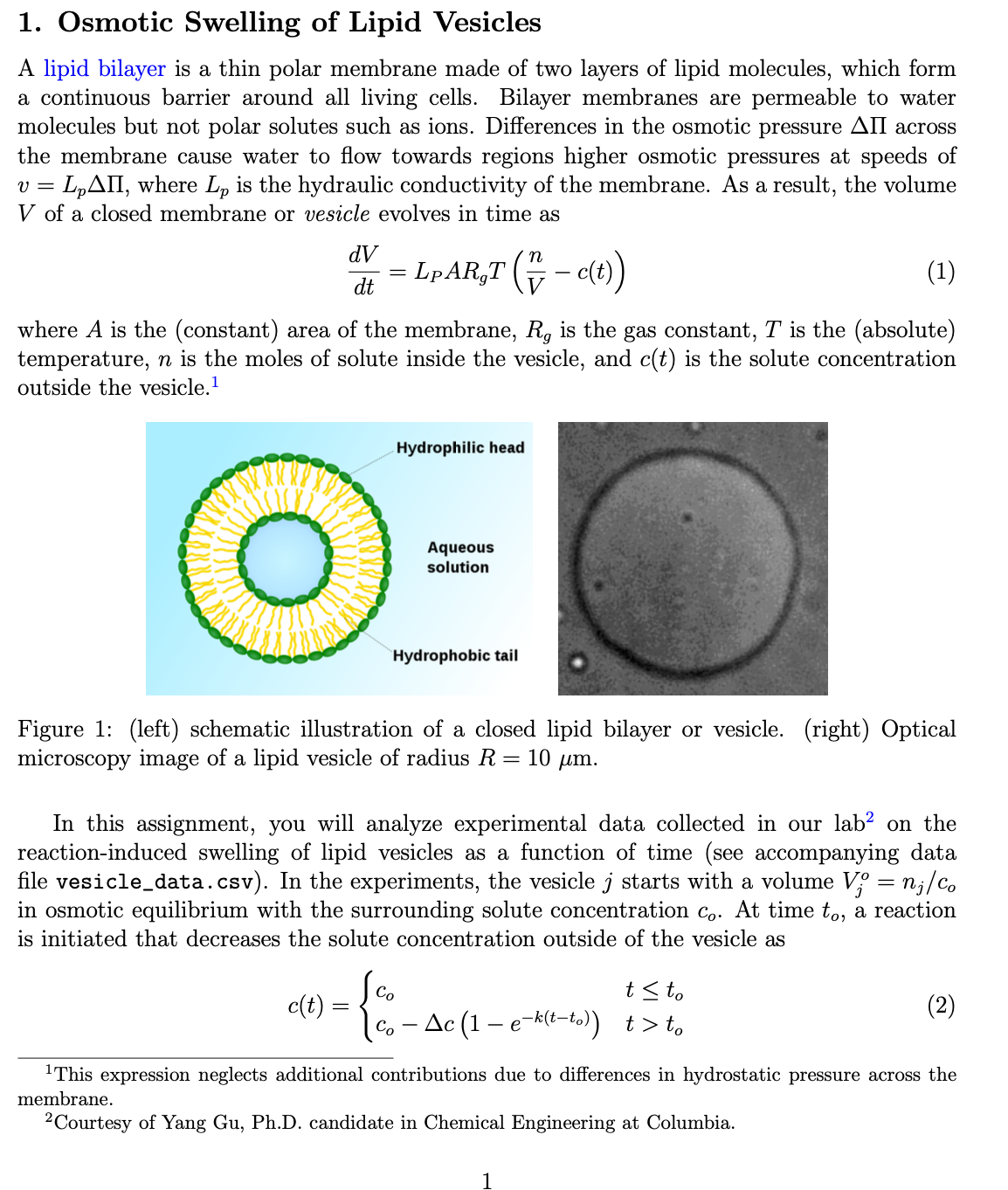

1. Osmotic Swelling of Lipid Vesicles A lipid bilayer is a thin polar membrane made of two layers of lipid molecules, which form a continuous barrier around all living cells. Bilayer membranes are permeable to water molecules but not polar solutes such as ions. Differences in the osmotic pressure AII across the membrane cause water to flow towards regions higher osmotic pressures at speeds of v = = LA, where Lp is the hydraulic conductivity of the membrane. As a result, the volume V of a closed membrane or vesicle evolves in time as dV dt = LP ART (- c(t)) (1) where A is the (constant) area of the membrane, R, is the gas constant, T is the (absolute) temperature, n is the moles of solute inside the vesicle, and c(t) is the solute concentration outside the vesicle. Hydrophilic head Aqueous solution Hydrophobic tail Figure 1: (left) schematic illustration of a closed lipid bilayer or vesicle. (right) Optical microscopy image of a lipid vesicle of radius R = 10 m. In this assignment, you will analyze experimental data collected in our lab on the reaction-induced swelling of lipid vesicles as a function of time (see accompanying data file vesicle_data.csv). In the experiments, the vesicle j starts with a volume V; = n;/co in osmotic equilibrium with the surrounding solute concentration co. At time to, a reaction is initiated that decreases the solute concentration outside of the vesicle as Co c(t): t to Co - Ac (1 ek(t-to)) t>to (2) This expression neglects additional contributions due to differences in hydrostatic pressure across the membrane. 2 Courtesy of Yang Gu, Ph.D. candidate in Chemical Engineering at Columbia. where Ac and k describe the magnitude and rate of the decrease. For small concentration changes (Acco), the volume of vesicle j is predicted to be Vi(t) { t to { + V [ (1 eask(t-to)) _ (1-e-k(t-to)) t> to V; - where = Ac/c is the fractional change in the solute concentration, and a; = LpA;RgTco/kV; is a dimensionless parameter that characterizes the relative rates of osmotic swelling and chemical reaction. We will consider two models for the vesicle radius R; (t) that correspond to limiting cases of equation (3) for slow reactions (a; > 1) and fast reactions (a; < 1) = tto R+R (1 ek(t-to)) t> to Ro Model A: R(t) = [R Model B: R;(t) = (4) t to - e e-K (t-to)/R) t> to where K = = 3L,R,Tc is a rate parameter for osmotic swelling. Note that three parameters , to, k in Model A and B, to, K in Model B- -are common to all vesicles j 1,..., M. By contrast, each vesicle j is characterized by its initial radius R; (this unknown parameter is not to be confused with the observed radius of vesicle j at time zero, which is subject to measurement error). Given data D = {ti, Ri,1, Ri,2} for the measured radii of M = 2 vesicles at N time points, your objective is to determine which of the two models is more probable and by how much. The measured radii are assumed to deviate from their predicted values due to additive Gaussian noise with standard deviation . For each model, (a) Compute the most probable parameter values and the associated error-bars. (b) Use your parameter estimates to plot the posterior prediction, R;(t) R%, along with the experimental data. (c) Estimate the the model likelihood p(D | M). Clearly state any assumptions that you make regarding the prior distributions for the pa- rameters.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


