Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Positronium is the given name of an exotic atom containing an electron and a positron (or anti-electron) moving around a common center of
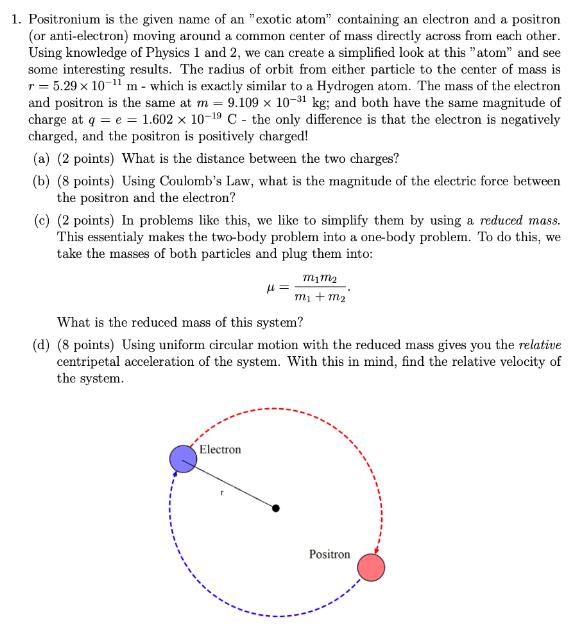
1. Positronium is the given name of an "exotic atom" containing an electron and a positron (or anti-electron) moving around a common center of mass directly across from each other. Using knowledge of Physics 1 and 2, we can create a simplified look at this "atom" and see some interesting results. The radius of orbit from either particle to the center of mass is r = 5.29 x 10- m - which is exactly similar to a Hydrogen atom. The mass of the electron and positron is the same at m = 9.109 x 10-1 kg; and both have the same magnitude of charge at q = e = 1.602 x 10-9 C- the only difference is that the electron is negatively charged, and the positron is positively charged! (a) (2 points) What is the distance between the two charges? (b) (8 points) Using Coulomb's Law, what is the magnitude of the electric force between the positron and the electron? (c) (2 points) In problems like this, we like to simplify them by using a reduced mass. This essentialy makes the two-body problem into a one-body problem. To do this, we take the masses of both particles and plug them into: = Electron M1M m + m What is the reduced mass of this system? (d) (8 points) Using uniform circular motion with the reduced mass gives you the relative centripetal acceleration of the system. With this in mind, find the relative velocity of the system. Positron
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions Since the radius is equivalent to the radius of hydrogen atom an...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


