Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Radiative forcing of greenhouse gases (20 points) The radiative forcings AFCH4 and AFN20, in W/m, caused by changes in the atmospheric concentrations of
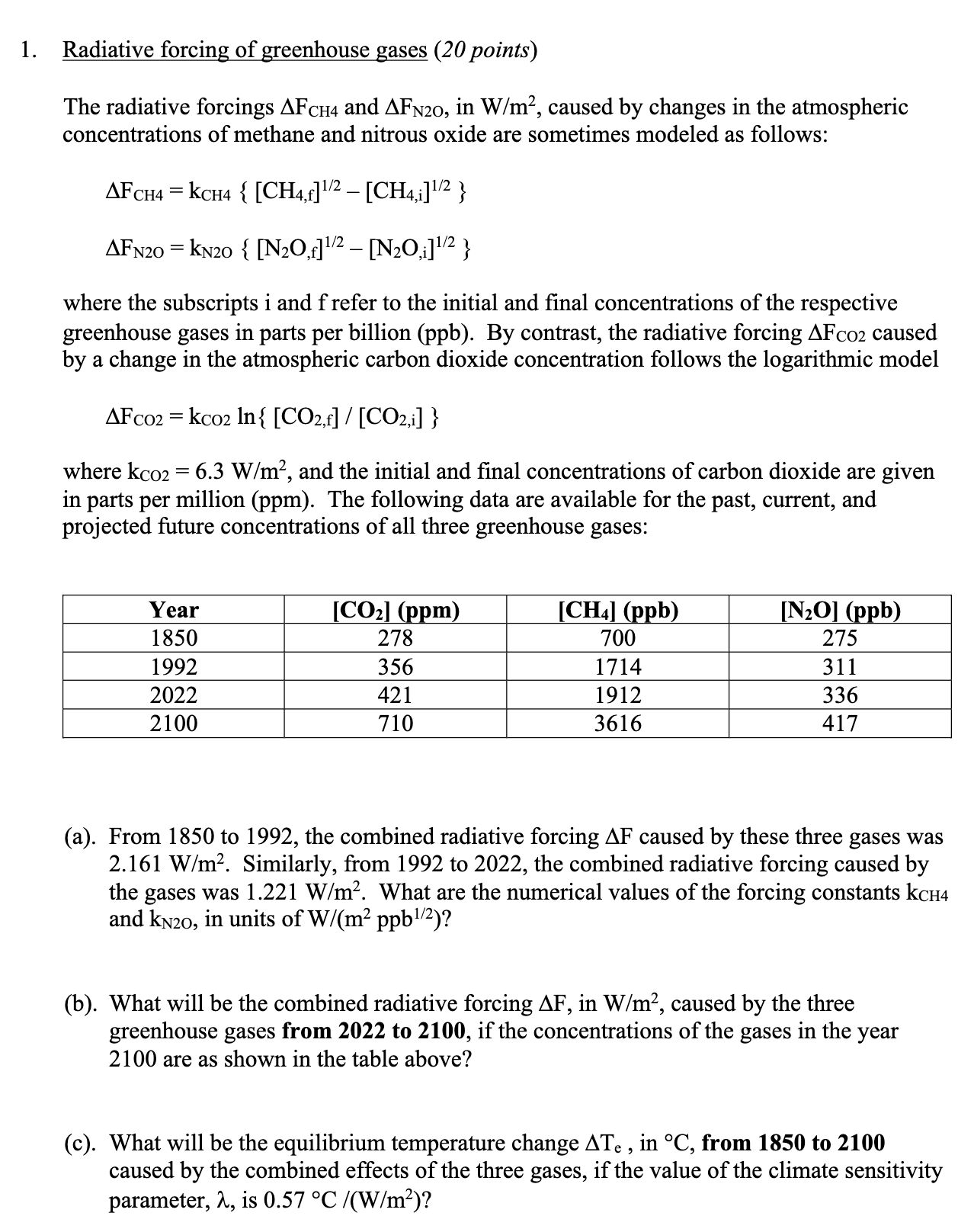
1. Radiative forcing of greenhouse gases (20 points) The radiative forcings AFCH4 and AFN20, in W/m, caused by changes in the atmospheric concentrations of methane and nitrous oxide are sometimes modeled as follows: AFCH4 = KCH4 { [CH4,f] /2 [CH4,i]/2} AFN20 = KN20 { [N2O,] 1/2 [NO,i] 1/2 } where the subscripts i and f refer to the initial and final concentrations of the respective greenhouse gases in parts per billion (ppb). By contrast, the radiative forcing AFco2 caused by a change in the atmospheric carbon dioxide concentration follows the logarithmic model = AFC02 kco2 In{ [CO2,f] / [CO2,i] } where kco2 = 6.3 W/m, and the initial and final concentrations of carbon dioxide are given in parts per million (ppm). The following data are available for the past, current, and projected future concentrations of all three greenhouse gases: Year 1850 [CO2] (ppm) [CH4] (ppb) [N2O] (ppb) 278 700 275 1992 356 1714 311 2022 421 1912 336 2100 710 3616 417 (a). From 1850 to 1992, the combined radiative forcing AF caused by these three gases was 2.161 W/m. Similarly, from 1992 to 2022, the combined radiative forcing caused by the gases was 1.221 W/m. What are the numerical values of the forcing constants kCH4 and KN20, in units of W/(m ppb 1/2)? (b). What will be the combined radiative forcing AF, in W/m, caused by the three greenhouse gases from 2022 to 2100, if the concentrations of the gases in the year 2100 are as shown in the table above? (c). What will be the equilibrium temperature change ATe, in C, from 1850 to 2100 caused by the combined effects of the three gases, if the value of the climate sensitivity parameter, , is 0.57 C /(W/m)?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


