Question
(a) In an Argon plasma, the argon pressure is 7.7 torr at 350 K, and the electron density is 4.3 x 1012 cm3. Assuming
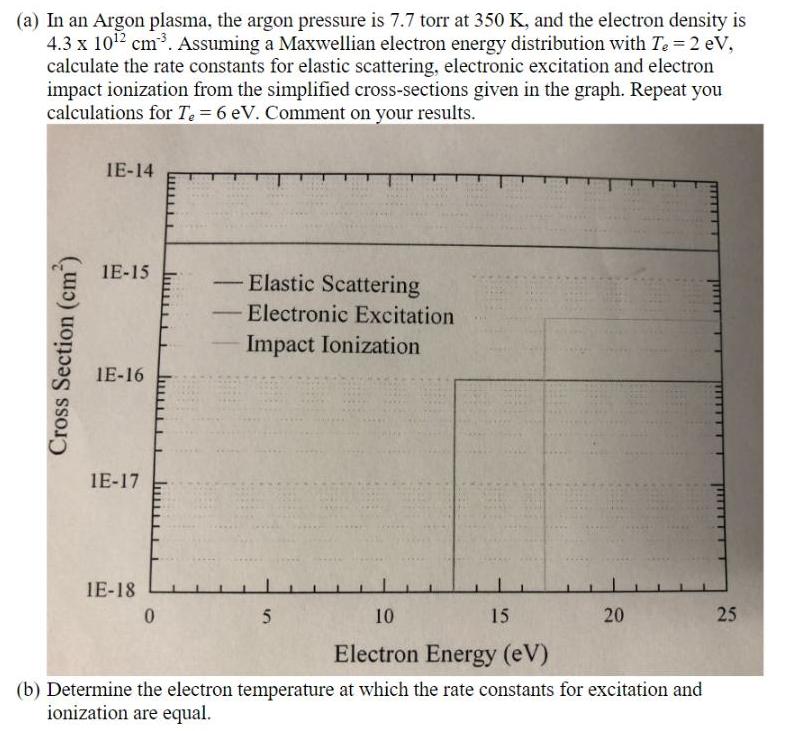
(a) In an Argon plasma, the argon pressure is 7.7 torr at 350 K, and the electron density is 4.3 x 1012 cm3. Assuming a Maxwellian electron energy distribution with Te 2 eV, calculate the rate constants for elastic scattering, electronic excitation and electron impact ionization from the simplified cross-sections given in the graph. Repeat you calculations for Te = 6 eV. Comment on your results. IE-14 1E-15 Elastic Scattering - Electronic Excitation - Impact Ionization IE-16 1E-17 IE-18 10 15 20 25 Electron Energy (eV) (b) Determine the electron temperature at which the rate constants for excitation and ionization are equal. Cross Section (cm)
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Rate Cooficients and Co s Sactions a For vamy genekal olision fuocess the ecte coefficient camy ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Physics
Authors: Jearl Walker, Halliday Resnick
8th Extended edition
471758019, 978-0471758013
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



