Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Starting out on a trip into the mountains, you inflate the tires with N2 (assuming ideal gas) on your automobile to a recommended

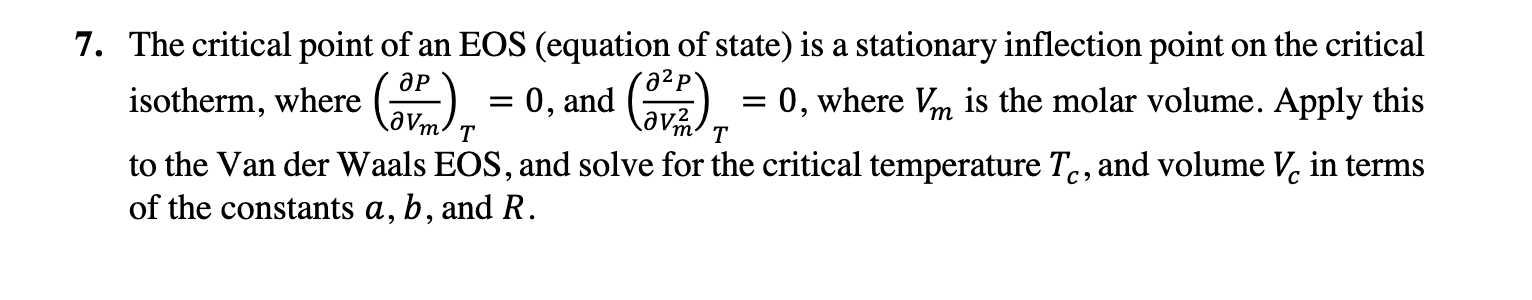
1. Starting out on a trip into the mountains, you inflate the tires with N2 (assuming ideal gas) on your automobile to a recommended pressure of 3.21 105 Pa on the day when the temperature is -5.00 C. You then drive to the beach, where the temperature is 28.0 C. (a) What is the final pressure in the tires, assuming constant volume? (b) Derive a formula for the final pressure, assuming more realistically that the volume of the tires increases with increasing pressure as V = V[1 + y(P P;)] where y is an experimentally determined constant. - (c) Show that the result in (b) has the correct limit as y 0, i.e., constant-V case in (a). 2. A seventeenth century physicist built a water barometer that projected through a hole in the roof of his house so that his neighbors could predict the weather by the height of the water. Suppose that a mercury barometer at 25 C reads 30.0 in, and the densities of mercury and water at 25 C are 13.53 and 1.00 g/cm respectively. What would the corresponding height of the column of water in the water barometer? 3. A certain mixture of He and Ne in a 356 cm bulb weighs 0.1480 g and is at 20.0 C and 748 torr. Find the mass and mole fraction of He present. 4. Pressure changes of an ideal gas. (a) For an ideal gas equation of state, prove that P P P dP == n - dn + dT T - dv. V (b) Suppose 1.000 mol of ideal gas at 300.0 K in a 30.00 L vessel increased in volume by 0.050 L. Use your answer from part (a) to compute the change in the pressure AP. (c) Compare to the exact change in pressure using the ideal gas equation of state to the estimate given by AP. 1 -1 5. For H2O at 17 C and 1 atm, = 1.7 10-4 K- and T = 4.7 10-5 atm- . A closed, rigid container is completely filled with liquid water at 14 C and 1 atm. If the temperature is raised to 20 C, estimate the pressure in the container. Neglect the pressure and temperature dependences of a and K. 6. A hypothetical gas obeys an equation of state PV = nRT (1 + CP), where c is a real constant. For this gas: (a) Show that = (b) Verify (or) V,n 1 = 1 and KT = P(1+cP) KT 7. The critical point of an EOS (equation of state) is a stationary inflection point on the critical isotherm, where (v) = 0, and (v) = 0, where Vm is the molar volume. Apply this T T to the Van der Waals EOS, and solve for the critical temperature Tc, and volume V in terms of the constants a, b, and R.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


