Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) Suppose we have the following combustion reaction C3H8+O2CO2+H2O All the parameters you can choose by yourself. a) From the left side we enter fuel,
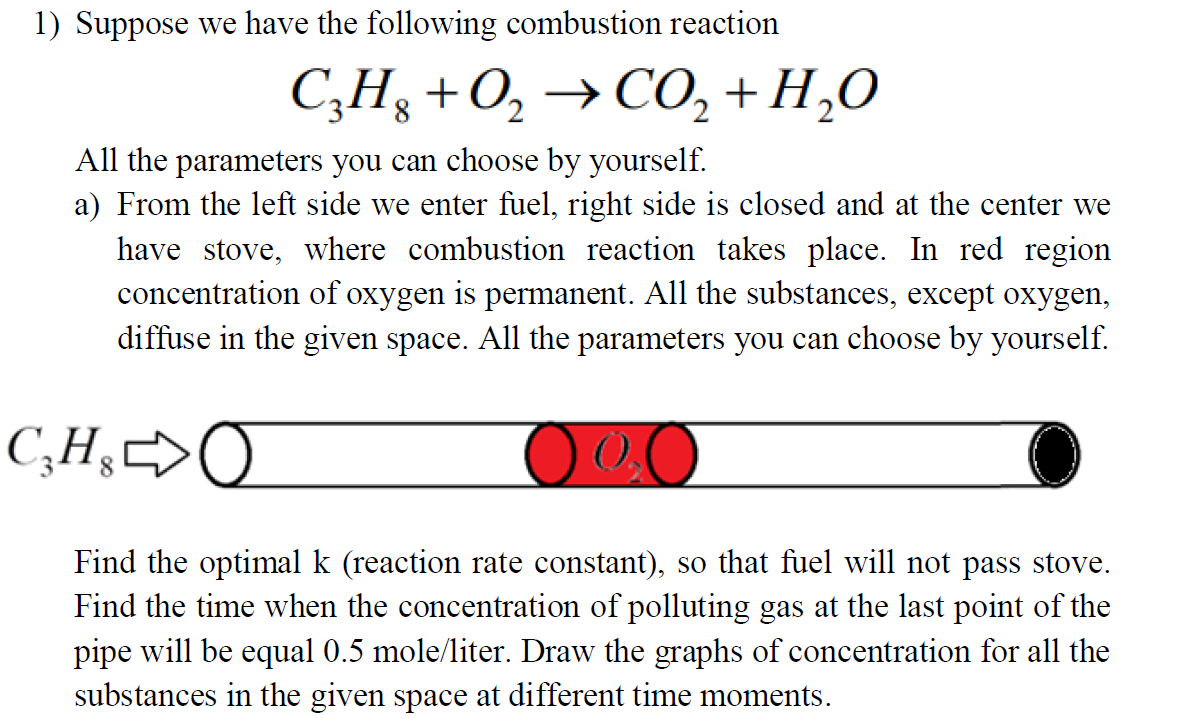
 1) Suppose we have the following combustion reaction C3H8+O2CO2+H2O All the parameters you can choose by yourself. a) From the left side we enter fuel, right side is closed and at the center we have stove, where combustion reaction takes place. In red region concentration of oxygen is permanent. All the substances, except oxygen, diffuse in the given space. All the parameters you can choose by yourself. Find the optimal k (reaction rate constant), so that fuel will not pass stove. Find the time when the concentration of polluting gas at the last point of the pipe will be equal 0.5 mole/liter. Draw the graphs of concentration for all the substances in the given space at different time moments. b) From the left side we enter the reagents, right side is open. All the substances diffuse in the given space. All the parameters you can choose by yourself. Find the number of iteration to reach the steady state. Draw the graphs of concentration for all the substances in the given space at different time moments
1) Suppose we have the following combustion reaction C3H8+O2CO2+H2O All the parameters you can choose by yourself. a) From the left side we enter fuel, right side is closed and at the center we have stove, where combustion reaction takes place. In red region concentration of oxygen is permanent. All the substances, except oxygen, diffuse in the given space. All the parameters you can choose by yourself. Find the optimal k (reaction rate constant), so that fuel will not pass stove. Find the time when the concentration of polluting gas at the last point of the pipe will be equal 0.5 mole/liter. Draw the graphs of concentration for all the substances in the given space at different time moments. b) From the left side we enter the reagents, right side is open. All the substances diffuse in the given space. All the parameters you can choose by yourself. Find the number of iteration to reach the steady state. Draw the graphs of concentration for all the substances in the given space at different time moments Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


