Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) The experimental data for liquid-liquid equilibrium in the CO:-1-decane system appear in the following table: T(K) P (bar) X' CO2 XCO 235.65 10.58 0.577
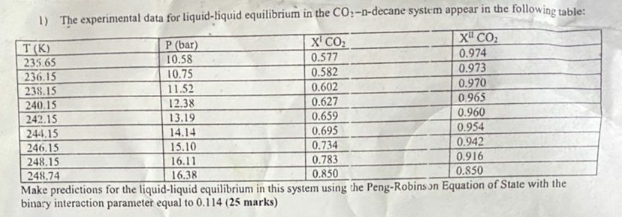
1) The experimental data for liquid-liquid equilibrium in the CO:-1-decane system appear in the following table: T(K) P (bar) X' CO2 XCO 235.65 10.58 0.577 0.974 236.15 10.75 0.582 0.973 238.15 11.52 0.602 0.970 240.15 12.38 0.627 0.965 242.15 13.19 0.659 0.960 244.15 14.14 0.695 0.954 246.15 15.10 0.734 0.942 248.15 16.11 0.783 0.916 248.74 16.3R 0.850 0.850 Make predictions for the liquid-liquid equilibrium in this system using the Peng-Robinson Equation of State with the binary interaction parameter equal to 0.114 (25 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


