Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. The following equation gives the analytic expression for entropy of an ideal gas, 5 S=KB = KN [In ( + ( 9) )
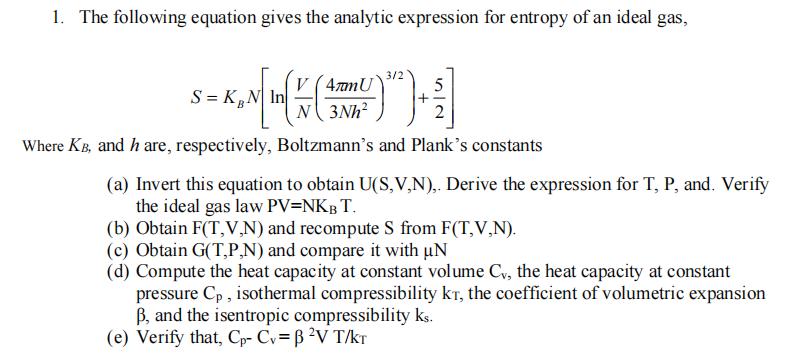
1. The following equation gives the analytic expression for entropy of an ideal gas, 5 S=KB = KN [In ( + ( 9) ") + 1/ 3/2 V (4mmU N3Nh 2 Where KB, and h are, respectively, Boltzmann's and Plank's constants (a) Invert this equation to obtain U(S,V,N),. Derive the expression for T, P, and. Verify the ideal gas law PV=NKB T. (b) Obtain F(T,V,N) and recompute S from F(T,V,N). (c) Obtain G(T,P,N) and compare it with un (d) Compute the heat capacity at constant volume Cv, the heat capacity at constant pressure Cp, isothermal compressibility kr, the coefficient of volumetric expansion B, and the isentropic compressibility ks. (e) Verify that, Cp- Cv = B V T/KT
Step by Step Solution
★★★★★
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
The question youve provided deals with thermodynamics specifically the analytical expression for the entropy S of an ideal gas and various thermodynamic functions derived from it Lets address each par...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


