Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. The freezing point and boiling point of acetone are 178.5 K and 329.20 K, respectively, or, -138.5F and 132.89F, or 321.21Ra and 592.56Ra
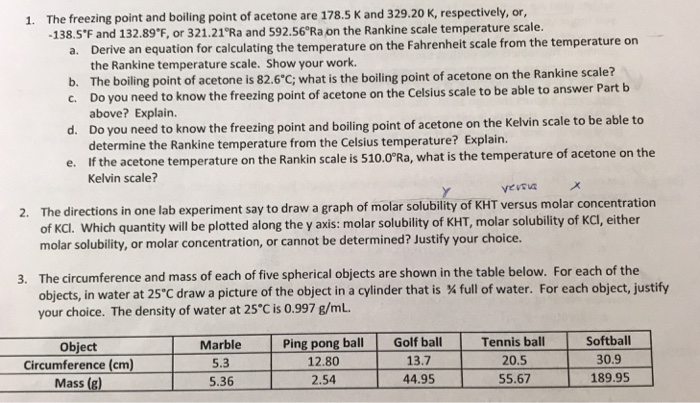
1. The freezing point and boiling point of acetone are 178.5 K and 329.20 K, respectively, or, -138.5F and 132.89F, or 321.21Ra and 592.56Ra on the Rankine scale temperature scale. a. Derive an equation for calculating the temperature on the Fahrenheit scale from the temperature on the Rankine temperature scale. Show your work. b. The boiling point of acetone is 82.6C; what is the boiling point of acetone on the Rankine scale? C. Do you need to know the freezing point of acetone on the Celsius scale to be able to answer Part b above? Explain. d. Do you need to know the freezing point and boiling point of acetone on the Kelvin scale to be able to determine the Rankine temperature from the Celsius temperature? Explain. e. If the acetone temperature on the Rankin scale is 510.0Ra, what is the temperature of acetone on the Kelvin scale? Y versva 2. The directions in one lab experiment say to draw a graph of molar solubility of KHT versus molar concentration of KCI. Which quantity will be plotted along the y axis: molar solubility of KHT, molar solubility of KCl, either molar solubility, or molar concentration, or cannot be determined? Justify your choice. 3. The circumference and mass of each of five spherical objects are shown in the table below. For each of the objects, in water at 25C draw a picture of the object in a cylinder that is % full of water. For each object, justify your choice. The density of water at 25C is 0.997 g/mL. Object Circumference (cm) Mass (g) Marble 5.3 5.36 Ping pong ball 12.80 2.54 Golf ball 13.7 44.95 Tennis ball 20.5 55.67 Softball 30.9 189.95
Step by Step Solution
★★★★★
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
1Fahranite and Rankine scale C F 32 59 1 Therefore we can convert F to C as K C 27315 2 Relati...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


