Question
Solid Lithium hydroxide and carbon dioxide gas react to produce aqueous lithium carbonate and water. What mass in g of lithium carbonate is produced
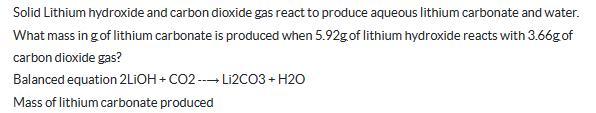
Solid Lithium hydroxide and carbon dioxide gas react to produce aqueous lithium carbonate and water. What mass in g of lithium carbonate is produced when 5.92g of lithium hydroxide reacts with 3.66g of carbon dioxide gas? Balanced equation 2LiOH + CO2-Li2CO3 + H2O Mass of lithium carbonate produced
Step by Step Solution
3.42 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1
Okay here are the steps to solve this problem 1 Balance...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Financial Accounting
Authors: Fred Phillips, Robert Libby, Patricia Libby
4th edition
978-0073369709, 73369705, 78025370, 978-0077444846, 77444841, 978-0078025372
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



