Question
1. The molar enthalpy of a ternary mixture of species a, b, and c can be described by the following expression: a. hm =
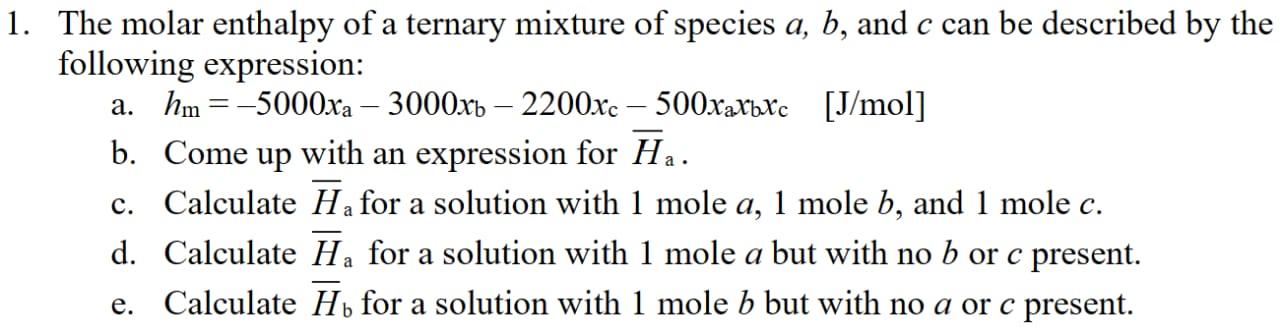
1. The molar enthalpy of a ternary mixture of species a, b, and c can be described by the following expression: a. hm = -5000xa - 3000xb - 2200xc-500xaxbxc [J/mol] b. Come up with an expression for Ha. c. Calculate Ha for a solution with 1 mole a, 1 mole b, and 1 mole c. d. Calculate Ha for a solution with 1 mole a but with no b or c present. e. Calculate Hb for a solution with 1 mole b but with no a or c present.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Part a The enthalpy of the mixture H in Joules can be expressed using the following equation H 5000 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
4th edition
978-1118431221, 9781119192138, 1118431227, 1119192137, 978-1119498759
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



