Question
(1) The volume of the cylinder was 1L at 50 C. What will be the new volume Vat T=100 C? Assume that the mass
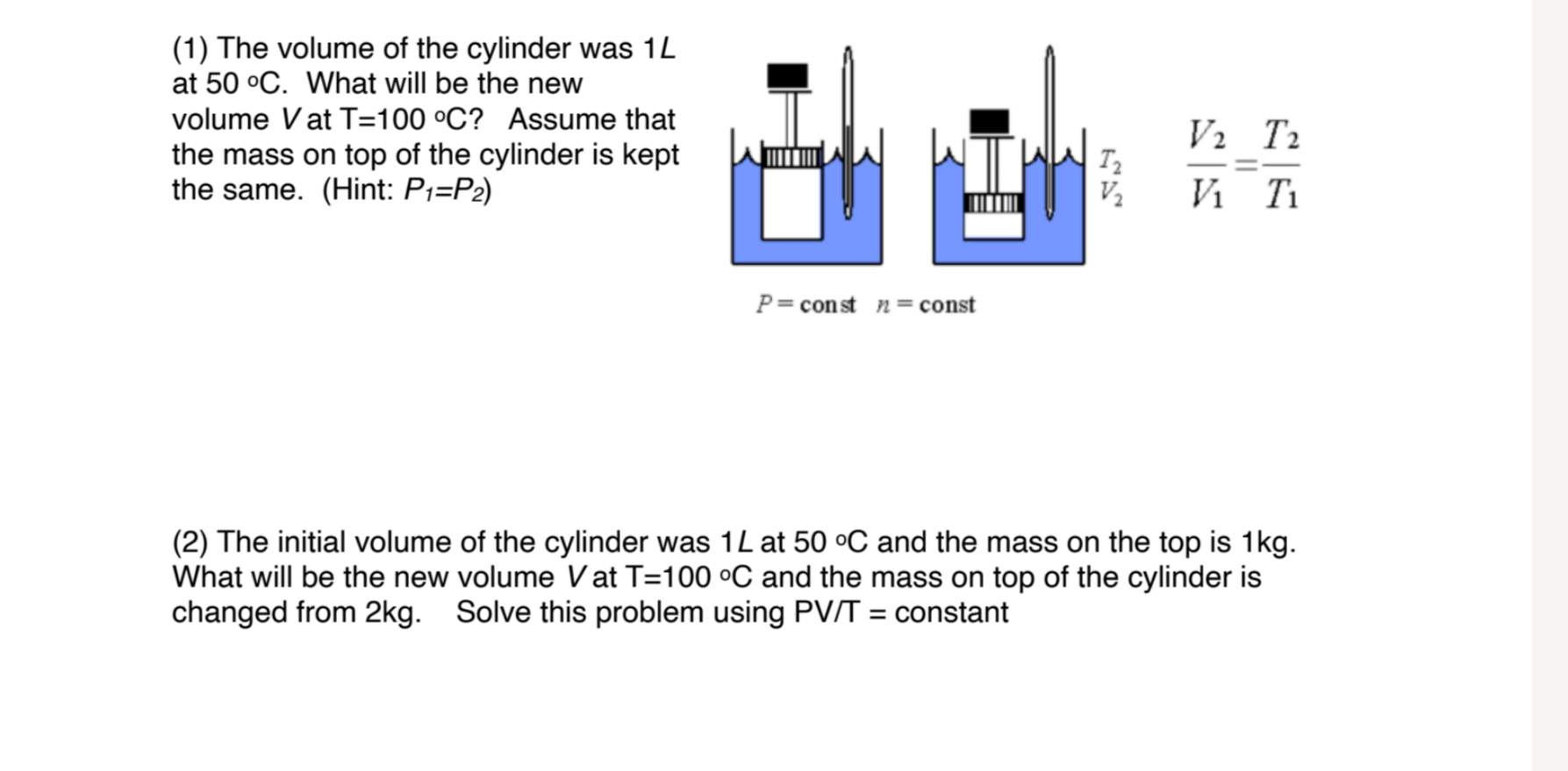
(1) The volume of the cylinder was 1L at 50 C. What will be the new volume Vat T=100 C? Assume that the mass on top of the cylinder is kept the same. (Hint: P1=P2) P=const n=const V2 T2 = V V Ti (2) The initial volume of the cylinder was 1L at 50 C and the mass on the top is 1kg. What will be the new volume V at T=100 C and the mass on top of the cylinder is changed from 2kg. Solve this problem using PV/T = constant
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



